 |
 |
 |
| |
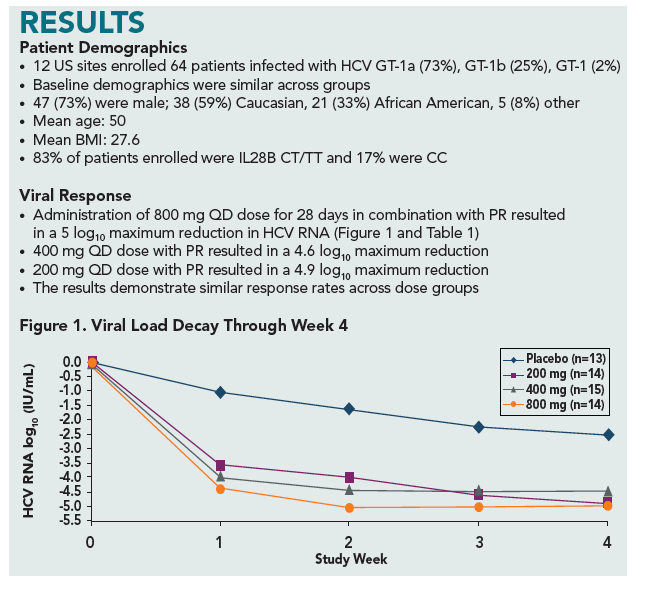
HIGH RAPID VIROLOGIC RESPONSE (RVR) WITH ACH-1625 DAILY DOSING PLUS PEGIFN-
ALPHA 2A/RBV IN A 28-DAY PHASE 2A TRIAL
|
| |
| |
Reported by Jules Levin
AASLD Nov 5-9 2011 SF
Jacob Lalezari1, Lydie Hazan2, Martin Kankam3, Eric Lawitz4, Fred Poordad5,
Victor Araya6, Reem Ghalib7, Bruce Bacon8, Steven Flamm9, Eliot Godofsky10, Federico Hinestrosa11, Atul Agarwal12, Mingjun Huang12, Milind Deshpande12, Heather Robison12, Lisa Robarge12, Elizabeth Olek12
Affiliations: 1Quest Clinical Research, San Francisco, CA, 2Axis Clinical Trials, Los Angeles, CA, 3Vince and Associates Clinical Research, Overland Park, KS, 4Alamo Medical Research, San Antonio, TX, 5Cedars Sinai Medical Center, Los Angeles, CA, 6Albert Einstein Medical Center, Philadelphia, PA, 7The North Texas Research Institute, Arlington, TX, 8Saint Louis University of Medicine, Saint Louis, MO, 9Northwestern University, Chicago, IL, 10University Hepatitis Center, Sarasota, FL, 11Orlando Immunology Center, Orlando, FL, 12Achillion Pharmaceuticals, New Haven, CT
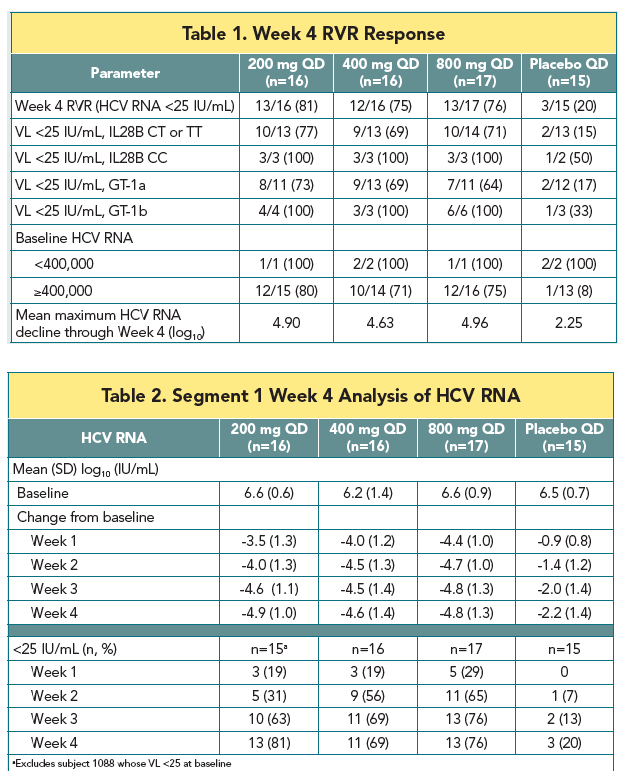
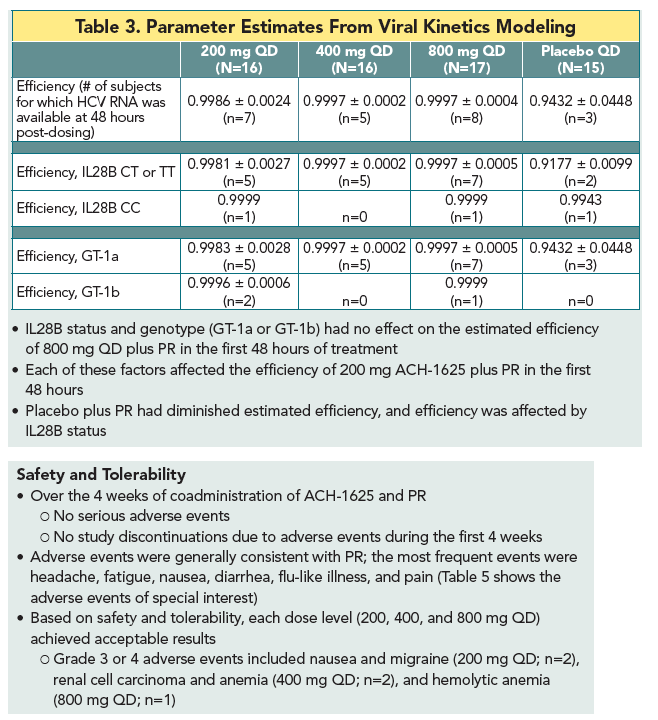
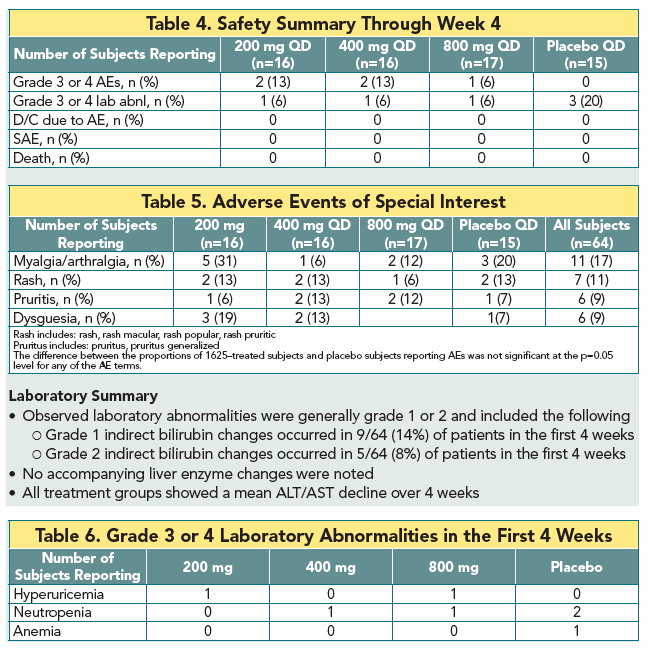

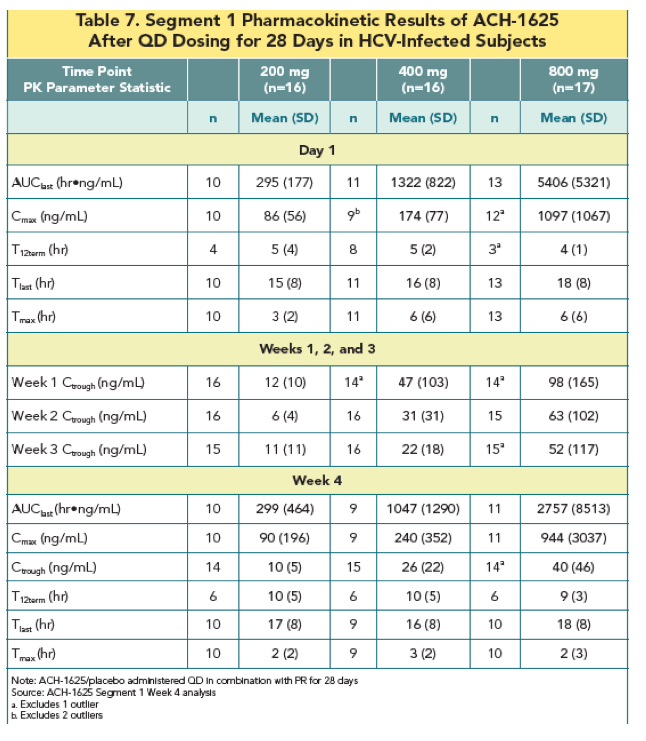
|
| |
|
 |
 |
|
|