 |
 |
 |
| |
12-Week Effiacy and Safety of ABT-072 or ABT-333 with Pegylated Interferon + Ribavirin, Following 3-Day Monotherapy in Genotype 1 HCV-Infected Treatment-Naïve Subjects
|
| |
| |
Reported by Jules Levin
Presented at 21st Conference of the Asian Pacifi c Association for the Study of the Liver (APASL 2011) · 17-20 February 2011
Queen Sirikit National Convention Center, Bangkok, Thailand
Isabelle A. Gaultier, Daniel E. Cohen, Emily O. Dumas, Lois M. Larsen, Thomas J. Podsadecki, Barry Bernstein
Global Pharmaceutical Research and Development, Abbott, Abbott Park, IL
BACKGROUND
· ABT-072 and ABT-333 are non-nucleoside HCV NS5B polymerase inhibitors being developed for the treatment of HCV genotype 1 infection in combination with other anti-HCV agents
· ABT-072 and ABT-333 were safe and well tolerated in single and multiple dose
studies in healthy volunteers1-3
· In a previous clinical trial, subjects receiving two days of ABT-072 monotherapy had a mean maximum HCV RNA decrease from baseline of 1.3 log10 IU/mL compared with 0.3 log10 IU/mL for placebo4
· In a second previous clinical trial, ABT-333 monotherapy for two days followed by 26 days of ABT-333 with pegylated interferon + ribavirin (P/R) resulted in significantly greater HCV RNA decreases compared with subjects that received P/R alone [3.73 log10 IU/mL vs. 1.37 log10 IU/mL, P=0.001]5
· We present here preliminary results at week 12 of a study of ABT-072 and ABT-333 in combination with P/R in genotype 1 HCV-infected treatment-naïve subjects
Objective
To analyze the efficacy and safety of various doses of once-daily ABT-072 or
twice-daily ABT-333 given for 3 days as monotherapy, followed by co-administration of ABT-072 or ABT-333 with P/R through 12 weeks of treatment
Methods
Study Design
· Study M11-602 (registered with ClinicalTrials.gov as NCT01074008) is an on-going randomized, placebo-controlled, blinded (active versus placebo within each arm), dose ranging, phase 2a clinical trial. In this study, subjects were randomized to receive various doses of 1 of 3 direct acting antiviral agents (DAA: ABT-072, ABT-333 or the HCV protease inhibitor ABT-450/r). All subjects have reached week 12 or discontinued study (Figure 1).
· Preliminary results from the fi rst 12 weeks of treatment with P/R combined with
ABT-072, ABT-333, or placebo are presented here. Data from the ABT-450/r
protease inhibitor-containing arms have been presented elsewhere.6, 7
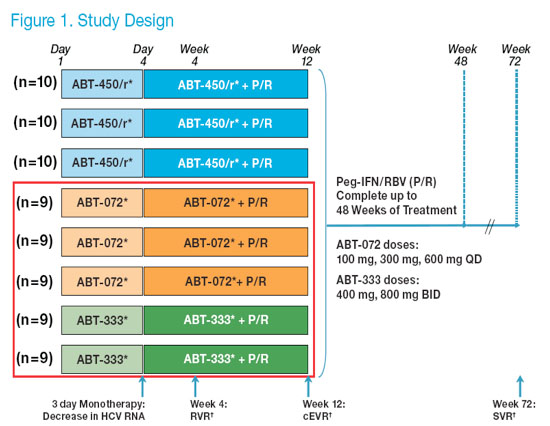
*Placebo Controlled: n=2 placebo per ABT-450/r group and n=1 placebo per ABT-072 and ABT-333 group.
†RVR: HCV RNA <25 IU/mL (LLOQ) at week 4, cEVR: HCV RNA <25 IU/mL (LLOQ) at week 12, SVR: HCV RNA <10 IU/mL (LLOD) at week 72.
To be eligible for enrollment, subjects had to meet the following inclusion criteria:
- age 18 to 65 years
- body mass index (BMI)>/= 18 and <35 kg/m2
- chronic HCV genotype 1 infection for at least 6 months prior to study enrollment
- plasma HCV RNA level >/=100,000 IU/mL at screening
- liver biopsy within the past 3 years with histology consistent with HCV-induced liver damage
Exclusion criteria included:
- liver biopsy with a METAVIR fi brosis score of 3 or 4
- positive test result for hepatitis B surface antigen or anti-HIV antibodies
- history of major depression within the 2 years prior to enrollment
- unresolved clinically signifi cant diseases other than HCV
Subjects were randomized to one of 3 once-daily doses of ABT-072 (100 mg, 300 mg, or 600 mg QD), one of 2 twice-daily doses of ABT-333 (400 mg or 800 mg BID), or placebo matched to active dose for 3 days
Following 3 days of monotherapy, subjects received ABT-072, ABT-333, or placebo at the same dose in combination with pegylated interferon alfa-2a 180 μg/week + weight-based ribavirin 1000-1200 mg/day (P/R) through week 12. At week 12, ABT-072, ABT-333, or placebo was discontinued and subjects received P/R alone through week 48.
Efficacy Analyses
· HCV RNA was measured using the Roche COBAS TaqMan® assay
(LLOQ = 25 IU/mL and LLOD = 10 IU/mL)
· Virologic response was assessed as HCV RNA decreas
e from baseline in
log10 IU/mL at each time point
· All subjects randomized to placebo in the study were pooled in this analysis
· Primary endpoint: Mean maximum decrease in HCV RNA during the 3-day
monotherapy period (through day 4 predose), compared between treatment groups and placebo using a one-way ANCOVA with treatment group as factor and baseline HCV RNA levels as covariate
· Additional endpoints:
- Proportion of subjects with HCV RNA <25 IU/mL, assessed at week 4 (protocol-defined rapid virologic response, RVR) and at week 12 (protocol-defined complete early virologic response, cEVR), with pair-wise comparisons to placebo performed using Fisher’s exact test
- Pair-wise comparisons to placebo in mean change from baseline in HCV RNA
to each time point through week 12, performed using ANCOVA with treatment
group as factor and baseline HCV RNA levels as covariate
RESULTS
Subject Disposition and Baseline Characteristics
Fifty subjects were randomized: 23 to 1 of 3 doses of ABT-072, 16 to 1 of 2 doses of ABT-333, and 11 to placebo
Nine subjects discontinued treatment before week 12: five met virologic failure
criteria, three were lost to follow-up, and one discontinued due to a peginterferon-related severe adverse event (pain). No subjects discontinued treatment due to DAA-related adverse events.
Demographic and baseline characteristics were similar between groups (Table 1)
- 72% of subjects overall were infected with genotype 1a HCV
- 82% of subjects overall had baseline HCV RNA >800,000 IU/mL
Protocol-defined virologic failure = HCV RNA decrease from baseline <1 log10 IU/mL at week 4; or virologic breakthrough (confirmed
detectable HCV RNA following a confirmed HCV RNA value below the LLOQ); or virologic rebound (confirmed increase in HCV RNA of
>/=1 log10 IU/mL from nadir)
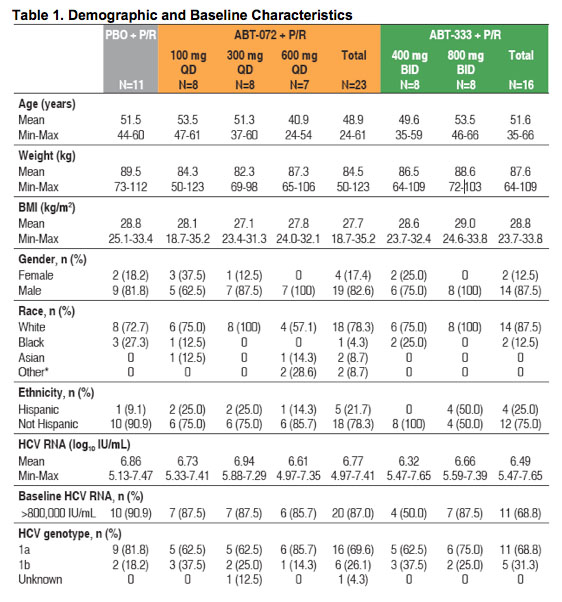
*Other = American Indian/Native Alaskan/Native Hawaiian or other Pacific Islander/Multi-race/Other.
Efficacy Results
Efficacy During 3-day Monotherapy
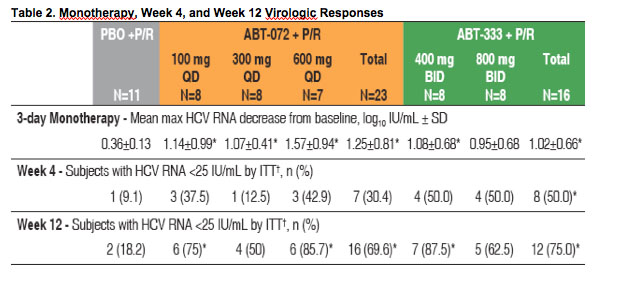
Through 3 days of monotherapy, subjects taking ABT-072 or ABT-333 had significantly greater mean maximum HCV RNA decreases (SD) from baseline than those receiving placebo (Table 2, Figure 2)
Figure 2. Mean HCV RNA Change From Baseline During 3-Day
Monotherapy by Dose Group (log10 IU/mL)
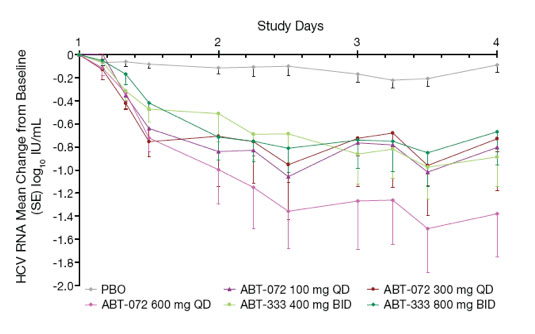
Two subjects contributed to a greater HCV RNA decrease in the ABT-072 600 mg
QD group: one subject with low baseline HCV RNA (5 log10 IU/mL) had a maximum decrease of 2.97 log10 IU/mL, and a subject infected with subtype 1b had a maximum decrease of 2.68 log10 IU/mL
Although samples sizes are small, there was a numerically greater HCV RNA decrease in subjects infected with subtype 1b than in subjects with subtype 1a for both ABT-072 (mean maximum decrease of 2.05 vs. 0.97) and ABT-333 (1.45 vs. 0.82) dose groups
Efficacy Through Week 12
· At week 12, when compared to subjects receiving placebo in combination with P/R,
a significantly higher proportion of subjects receiving active drug in combination with
P/R achieved cEVR: 69.6% (P=0.026) and 75% (P=0.006) in the ABT-072 and ABT-
333 dose groups, respectively (Table 2)
· One subject receiving ABT-072 300 mg + P/R experienced a virologic rebound
(HCV RNA increase >/=0.5 log10 IU/mL from nadir) during the 12 weeks of treatment.
No subject receiving ABT-333 + P/R experienced a virologic rebound.
Safety Results
Overall, ABT-072 and ABT-333 were well tolerated when administered alone and in combination with P/R
No DAA-related serious or severe adverse events were reported
Similar proportions of subjects reported at least one adverse event in all treatment
groups (Table 3)
Table 3. Most Commonly Reported Treatment-emergent Adverse Events
(>20%)
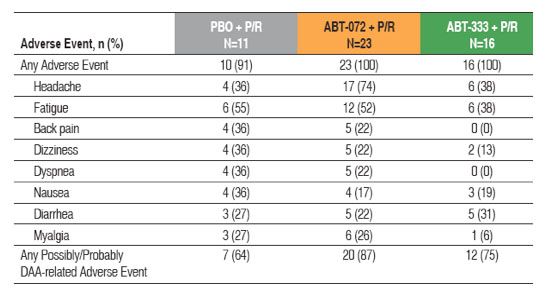
No relationship between dose and frequency of possibly or probably DAA-related
adverse events was observed for either ABT-072 or ABT-333 dose groups
Adverse events of at least moderate severity considered possibly or probably related to DAA by the investigator in more than 2 subjects are:
- Decrease of blood phosphorus: 0/11 (0%) on placebo + P/R compared to
2/23 (9%) on ABT-072 + P/R and 0/16 (0%) on ABT-333 + P/R
- Headache: 1/11 (9%) on placebo + P/R compared to 3/23 (13%) on
ABT-072 + P/R and 3/16 (19%) on ABT-333 + P/R
- Neutropenia: 0/11 (0%) on placebo + P/R compared to 1/23 (4.3%) on
ABT-072 + P/R and 2/16 (13%) on ABT-333 + P/R
Through week 12, the mean changes in neutrophil count were -3.65 X 109/L for
subjects receiving placebo + P/R, compared to -2.29 X 109/L for subjects receiving ABT-072 + P/R, and -2.03 X 109/L for subjects receiving ABT-333 + P/R. Neutropenia was managed by reduction of the pegIFN dose. No subject discontinued treatment for neutropenia
References
1. Dumas E et al. Pharmacokinetics of the HCV Polymerase Inhibitor ABT-072 Following Single and Multiple Dosing in Healthy Adult Volunteers. EASL 2010;14-18 April 2010; Vienna, Austria. Abstract #747.
2. Menon R et al. Pharmacokinetics and Tolerability of the HCV Polymerase Inhibitor ABT-333 Following Single Ascending Doses in Healthy Adult Volunteers. EASL 2009; 22-26 April 2009; Copenhagen, Denmark. Abstract #956.
3. Menon R et al. Pharmacokinetics, Safety and Tolerability of the HCV Polymerase Inhibitor ABT-333 Following Multiple Ascending Doses and Effect of Co-Administration of Ketoconazole in Healthy Subjects. EASL 2009; 22-26 April 2009; Copenhagen, Denmark. Abstract #957.
4. Klein CE et al. Safety, Tolerability, Pharmacokinetics and Antiviral Activity of the HCV Polymerase Inhibitor ABT-072 Following Single and Multiple Dosing in Healthy Adult Volunteers and Two Days of Dosing in Treatment-Naïve HCV Genotype 1-Infected Subjects. HepDART 2009; 06-10 December 2009; Kohala Coast, Hawaii, USA. Abstract#56.
5. Rodriguez-Torres M et al. Treatment-naïve, genotype-1 HCV-infected subjects show significantly greater HCV RNA decreases when treated with 28 days of ABT-333 plus peginterferon and ribavirin compared to peginterferon and ribavirin alone. AASLD 2009; 30 October-3 November; Boston, MA. Abstract #LB06
6. Lawitz E. et al. Initial antiviral activity of the HCV NS3 protease inhibitor ABT-450 when given with low dose ritonavir as 3-day monotherapy: Preliminary results of study M11-602 in genotype 1 (GT1) HCV-infected treatment-naïve subjects. AASLD 2010; 30 October 30-3 November; Boston, MA. Abstract #1855.
7. Lawitz E et al. 4-Week Virologic Response and Safety of ABT-450 Given with Low-dose Ritonavir (ABT-450/r) First As 3-Day Monotherapy then in Combination with Pegylated Interferon Alpha-2a and Ribavirin (SOC) in Genotype 1 (GT1) HCV-infected Treatment-naïve Subjects. AASLD 2010; 30 October 30-3 November; Boston, MA. Abstract #LB10.
|
| |
|
 |
 |
|
|