 |
 |
 |
| |
Sangamo's Gene Therapy for HIV is Safe, Helps Immune Cells in Early Trial
|
| |
| |
Bloomberg. CROI Feb 28, 2011
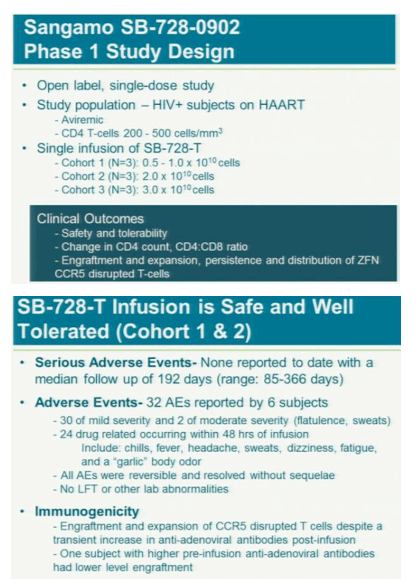
Sangamo Biosciences Inc.'s experimental gene therapy was used safely in six patients infected with HIV in the first trial of a new approach aimed at blocking the virus so patients won't need antiviral drugs.
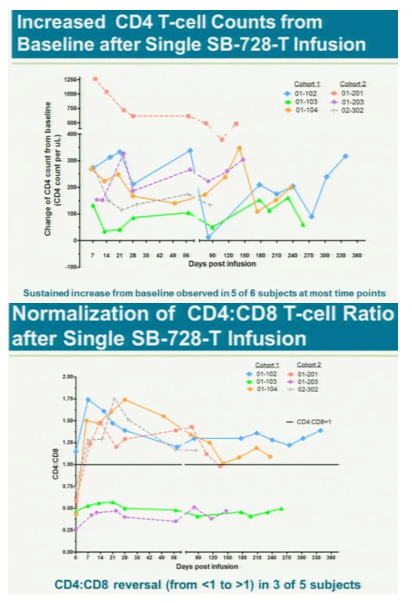
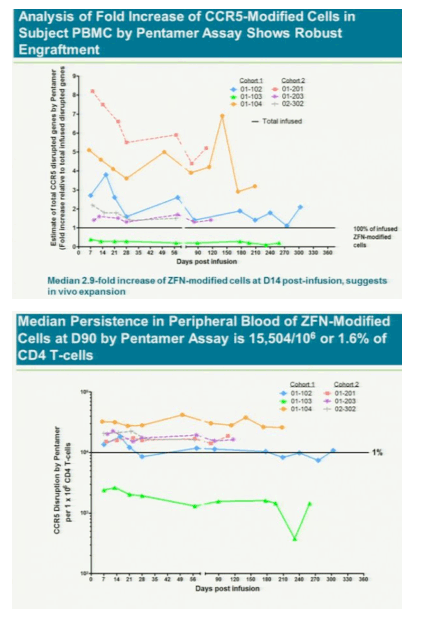
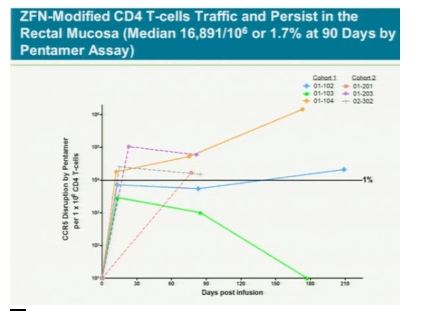
After a year, five of the six had "significant and largely sustained" increases in the number of infection-fighting t- cells in their system, said study leader Jacob Lalezari, director of Quest Clinical Research, a San Francisco clinical trial center. The patients kept taking drugs to suppress the virus during the study because the research first must assess whether the therapy is safe.
The treatment modifies patients' t-cells to disable a protein called CCR5 that HIV uses to enter the cells. Without the entry point, HIV might not be able to kill off the immune system cells and they will outlast or eventually overpower the virus, Lalezari said. If the therapy cuts HIV levels in patients who aren't taking antiviral drugs, it may gain approval by late 2013, said Liana Moussatos of Wedbush Securities in San Francisco.
"When that data comes at the end of this year, we should have an idea whether the efficacy is durable enough and potent enough," Moussatos, a biotechnology analyst, said in a Feb. 25 telephone interview. If approved, the drug may have sales of $750 million a year, she said.
from jules: Increased CD4 counts about 100. This is first clinical study showing such clinical benefit, however the true overall clinical benefit remains to be shown in future studies and analyses of the data
Webcasts and Podcasts
http://retroconference.org/2011/display.asp?page=460

|
| |
|
 |
 |
|
|