 |
 |
 |
| |
Raltegravir (RAL) Demonstrates Durable Virologic Suppression and Superior Immunologic Response with a Favorable Metabolic Profile Through 3 Years of Treatment (Tx): 156 Week (Wk) Results from STARTMRK
|
| |
| |
Reported by Jules Levin
CROI March 2 2011 Boston
J. K. Rockstroh,1 J. Lennox,2 E. DeJesus,3 M. Saag,4 A. Lazzarin,5 X. Xu,6 H. Teppler,6 B-Y. Nguyen,6 R. Leavitt,6 P. Sklar6 and the STARTMRK Study Team
1Univ. of Bonn, Bonn-Venusberg, Germany, 2Emory Univ., Atlanta, GA, USA 3Orlando Immunol Ctr, Orlando, FL, USA 4Univ. of Alabama at Birmingham, AL, USA, 5Univ. Vita-Salute San Raffaele, Milan, Italy, 6Merck Research Laboratories, North Wales, PA, USA
ABSTRACT
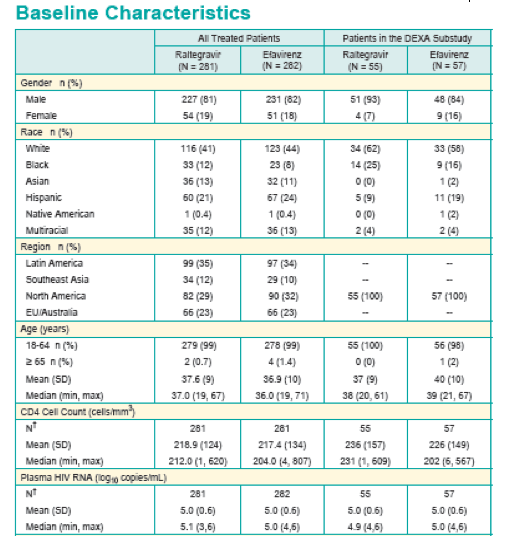
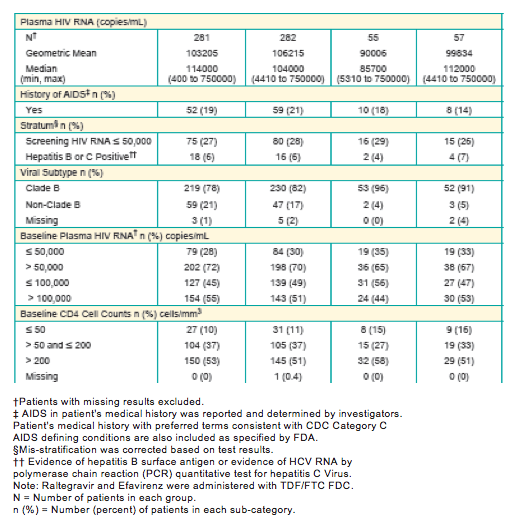
Background: As HIV tx has evolved to a paradigm of lifelong therapy, with greater relevance of co-morbidities, long-term data is essential to distinguish regimens. We report 156 Wk results from STARTMRK.
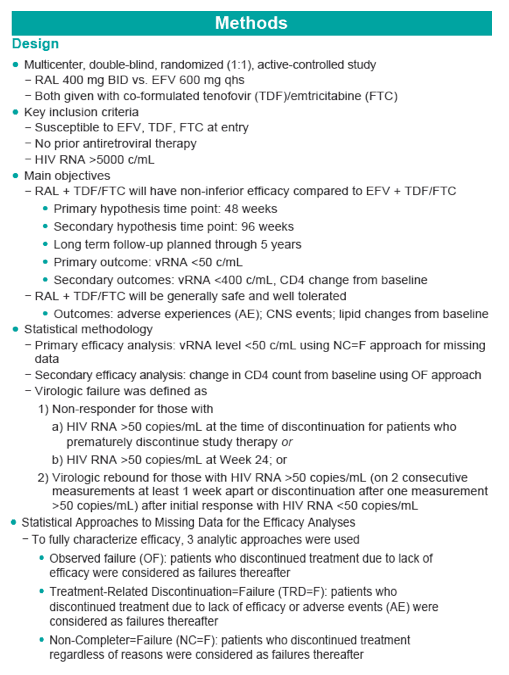
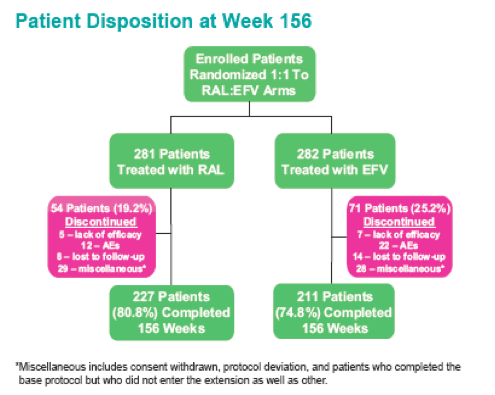
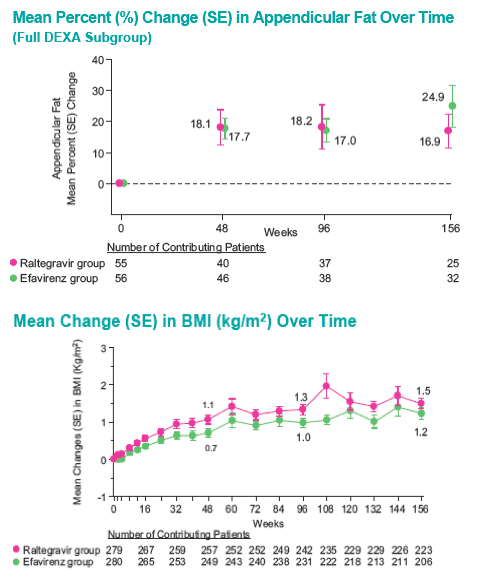
Methods: 563 Pts were randomized to RAL vs. EFV, each with TDF/FTC, in a double-blind study comparing standard efficacy endpoints and metabolic parameters. DEXA scans were obtained on a subset of Pts: 86 at baseline (BL) and Wk 48, 75 at BL and Wk 96, and 57 at BL and Wk 156. To fully characterize efficacy, 3 analytic approaches were used.
Results: Efficacy analyses at Wk 156 are summarized.
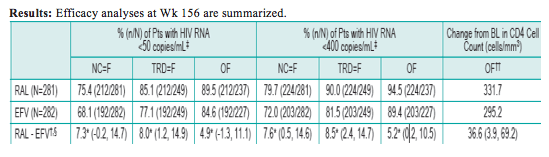
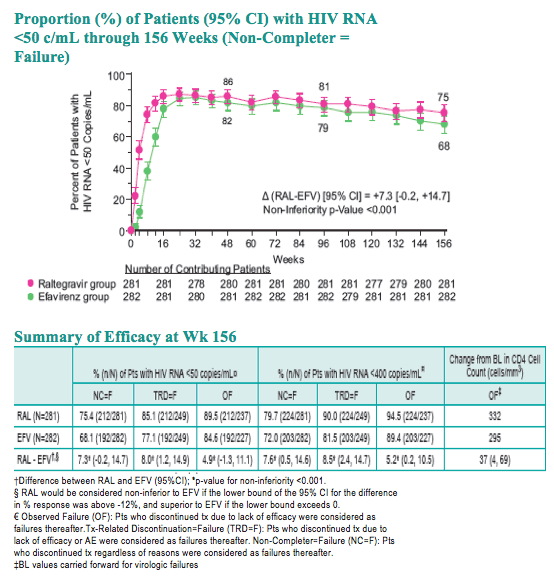
Difference between RAL and EFV (95%CI); *p-value for non-inferiority <0.001
RAL would be considered non-inferior to EFV if the lower bound of the 95% CI for the difference in % response was above -12%, and superior to EFV if the lower bound exceeds 0.
Observed Failure (OF): Pts who discontinued tx due to lack of efficacy were considered as failures thereafter.
Tx-Related Discontinuation=Failure (TRD=F): Pts who discontinued tx due to lack of efficacy or AE were considered as failures thereafter.
Non-Completer=Failure (NC=F): Pts who discontinued tx regardless of reasons were considered as failures thereafter.
BL values carried forward for virologic failures.
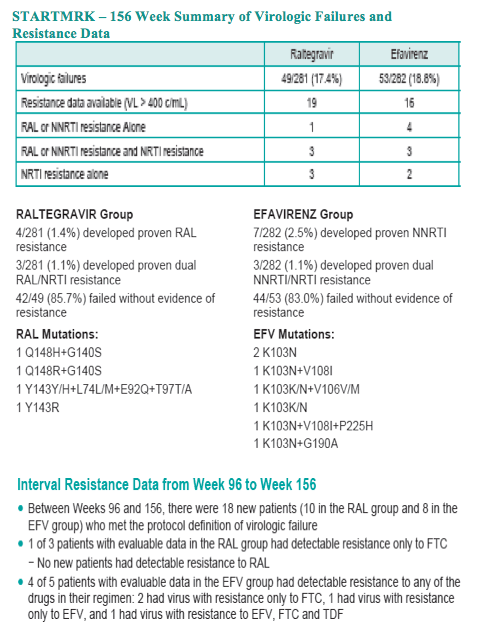
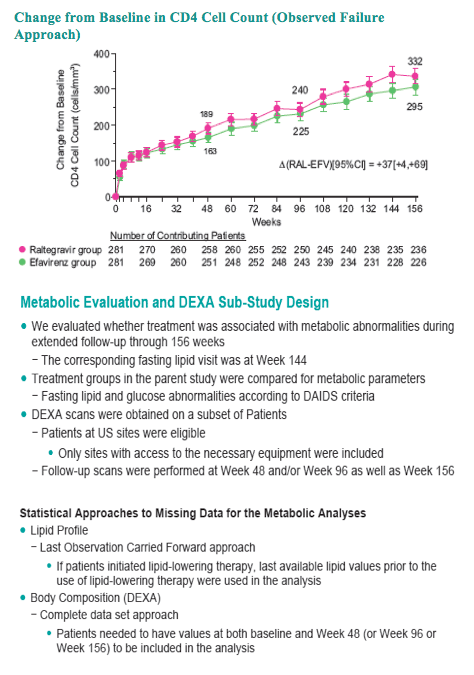
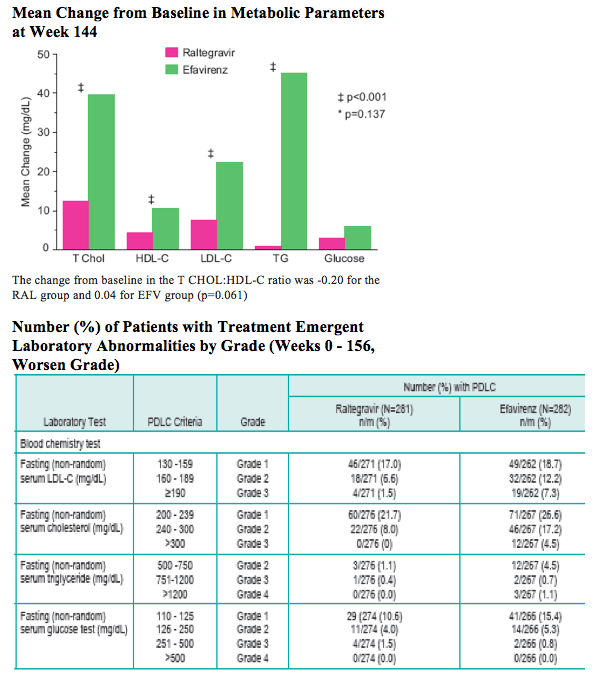
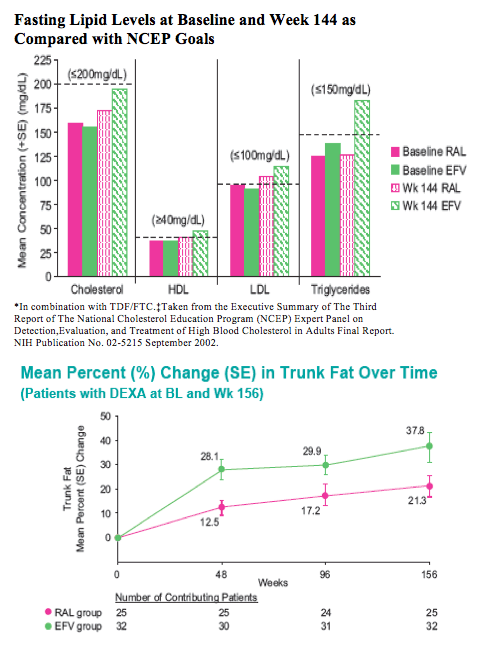
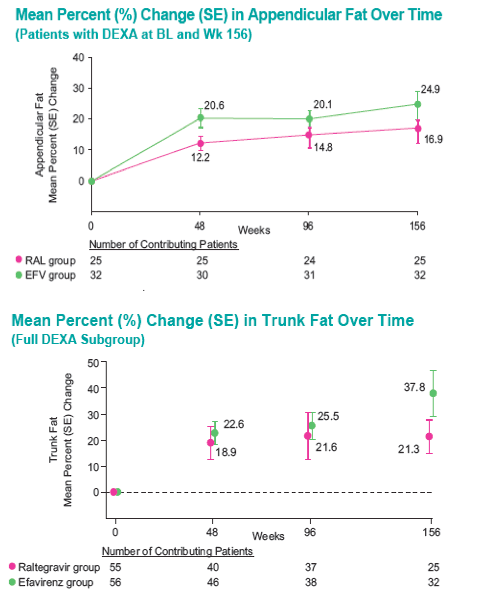
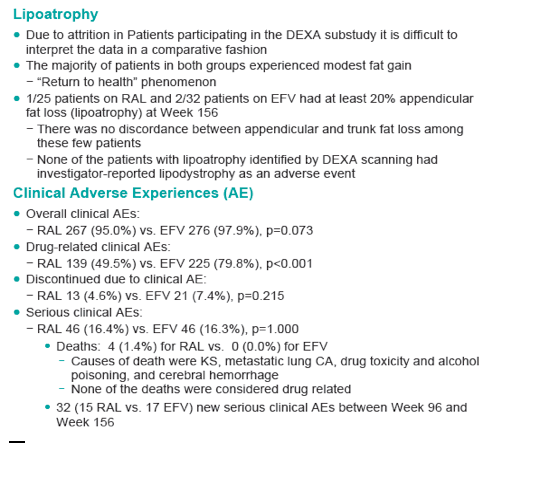
With longer-term follow-up, RAL demonstrates greater virologic suppression and immunologic response after 3 years of tx. Drug-related clinical AEs occurred less often with RAL than EFV (49% vs. 80%; p<0.001). RAL was generally well tolerated with few discontinuations due to AEs (5% RAL, 7% EFV). At Wk 156, RAL had less impact on fasting lipids than EFV. Fat changes by DEXA appeared numerically more favorable for RAL (Total Mean % Change, +19 RAL, +31 EFV) with no patterns of fat loss after 3 years of tx.
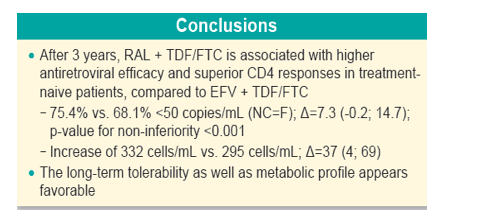
Conclusions: After 3 years, RAL + TDF/FTC is associated with higher antiretroviral efficacy and superior CD4 responses in tx-naive Pts. The long-term tolerability as well as metabolic profile appears favorable.
|
| |
|
 |
 |
|
|