 |
 |
 |
| |
Genotypic and Phenotypic Characterization of NS3 Variants Selected in
HCV-Infected Patients Treated with ABT-450
|
| |
| |
Reported by Jules Levin
EASL 2011 March 30-April 2 Berlin Germany
T.J. Pilot-Matias1, R.L. Tripathi1, T. Dekhtyar1, R.M. Menon2, I.A. Gaultier3, D.E. Cohen3, T.J. Podsadecki3, B.M. Bernstein3 and C.A. Collins1
1Antiviral Research, 2Clinical Pharmacokinetics and Pharmacodynamics, 3Pharmaceutical Development, Abbott Laboratories, Abbott Park, IL, USA
EASL: ABT-450/Ritonavir (ABT-450/r) Combined with Pegylated Interferon Alpha-2a and Ribavirin After 3-Day Monotherapy in Genotype 1 HCV-Infected Treatment-na´ve Subjects: 12-Week Interim Efficacy and Safety Results - (04/04/11)
BACKGROUND
ABT-450 is a potent HCV NS3/4A protease inhibitor identified as a lead compound by Abbott and Enanta
ABT-450 metabolism is primarily mediated by CYP3A4; therefore, co-administration of low-dose ritonavir (RTV) boosts the pharmacokinetics of ABT-450 and allows for once-daily dosing
The in vitro resistance profile of ABT-450 is similar to other NS3 protease inhibitors
In single and multiple dosing studies in healthy volunteers, adverse events were infrequent and generally mild
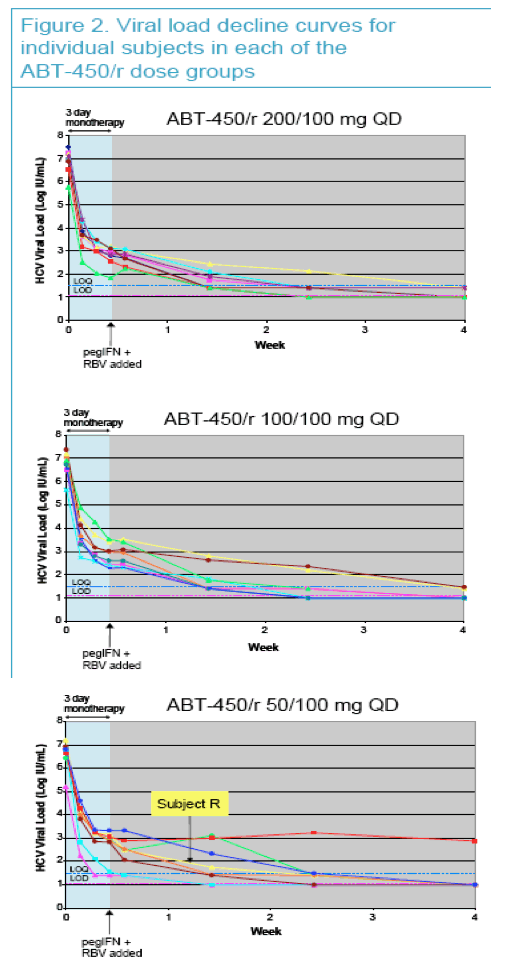
In the current phase 2a trial, ABT-450 (50, 100, 200 mg QD) was dosed with RTV 100 mg (ABT-450/r, n=8 per group) in GT 1 treatment-na´ve subjects for 3 days, followed by addition of pegIFN/RBV for a total of 12 weeks. Subjects then received up to 36 additional weeks of pegIFN/RBV therapy.
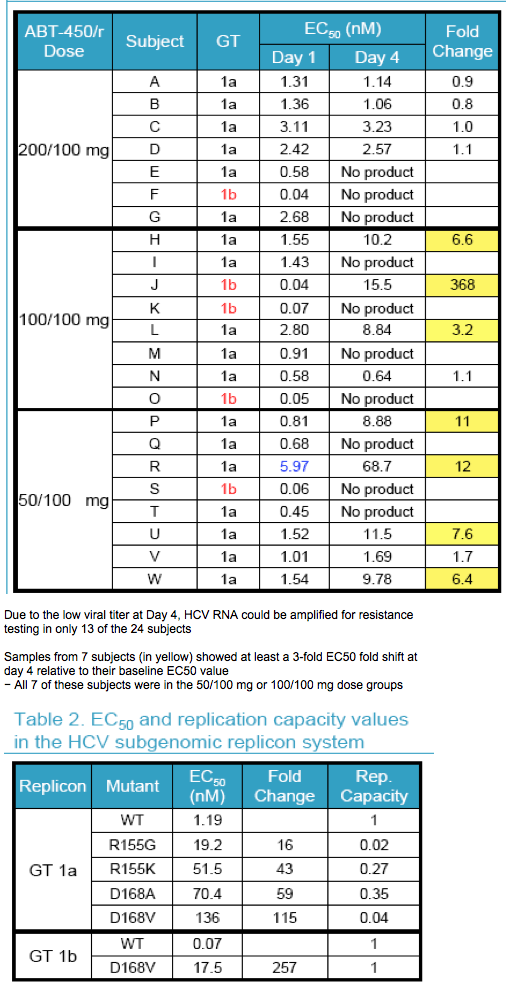
Mean maximum HCV RNA decrease was 4.02 log10 during the 3 day monotherapy, and 21 of 24 subjects had HCV RNA <25 IU/mL at 4 weeks
Objective
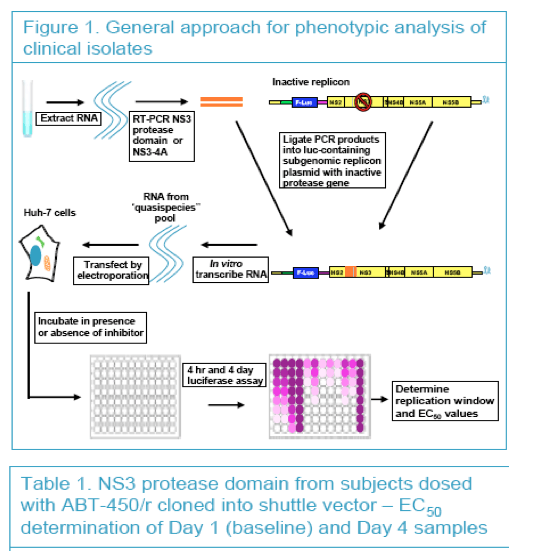
To assess the emergence of resistant mutants by genotypic and phenotypic analyses in conjunction with the kinetics of HCV RNA during the first 3 days of ABT-450/r dosing

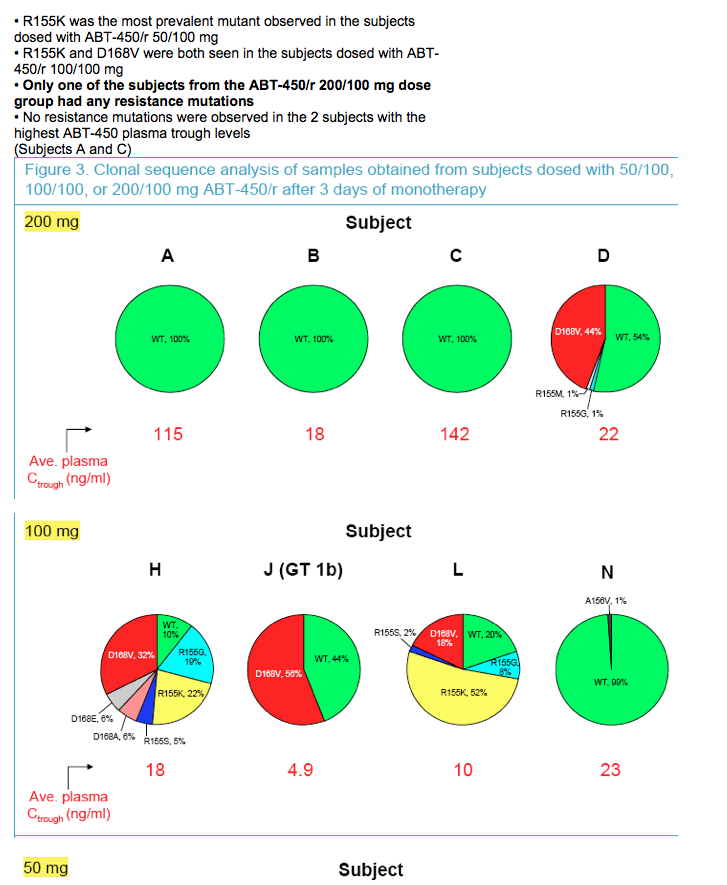
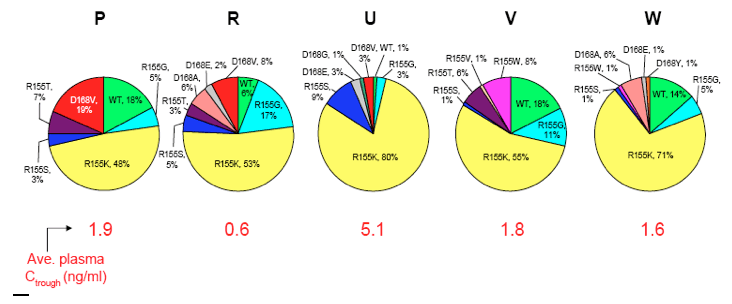
|
| |
|
 |
 |
|
|