 |
 |
 |
| |
GNS-227: A New Potent and Selective (2nd Gen)
HCV NS3 Protease Inhibitor With a High Genetic Barrier to Resistance
|
| |
| |
Reported by Jules Levin EASL 2011 Berlin Germany March 30-Apr 2
Philippe Halfon,1 Jérôme Courcambeck,1 Tony Whitaker,2 Mourad Bouzidi,1 Tamara R. McBrayer,2 Gilles Roche,1 Phillip M. Tharnish,2 Gérard Pèpe,1 Raymond F. Schinazi,3 and Steven J. Coats2
1Genoscience Pharma Marseille France, 2RFS Pharma LLC Tucker GA USA,
3EmoryUniversity/VA
Medical center Decatur GA USA
ABSTRACT
Background and aims: Future treatments of HCV are likely to include direct-acting antiviral agents that will improve the treatment options for HCV-infected individuals. Today, the major potential limitation of most protease inhibitors (PI) is a high risk for development of resistance.1 Thus, development of newer PI with high genetic barrier to resistance is warranted.
Methods: Based on structure-based drug design and HCV NS3 protease molecular resistance mechanisms, a set of peptidomimetic inhibitors of NS3/4A protease was synthesized and optimized. GNS-227 was identified to have high potency by optimizing its flexibility and interactions with the NS3 protease active site. Antiviral activity against genotype 1b HCV replicon, biochemical potency against wild type and PI mutants, inhibition against a panel of eight human proteases and four major human cytochrome P450 enzymes, and cytotoxicity was performed.
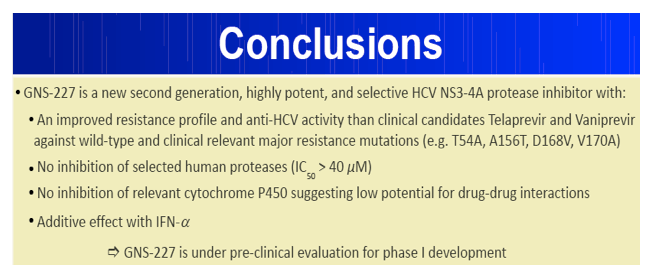
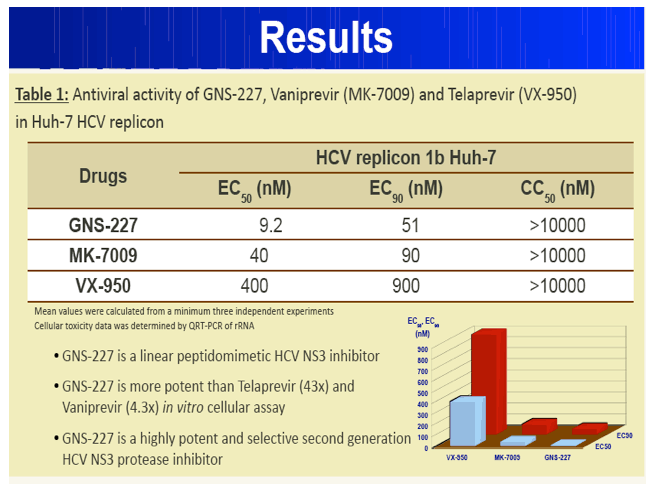
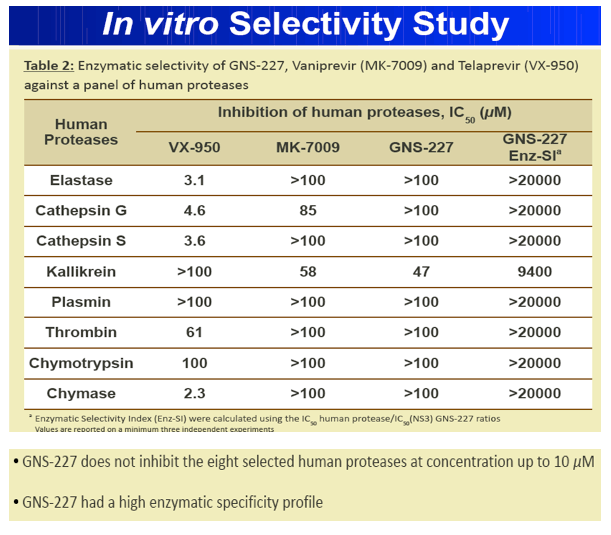
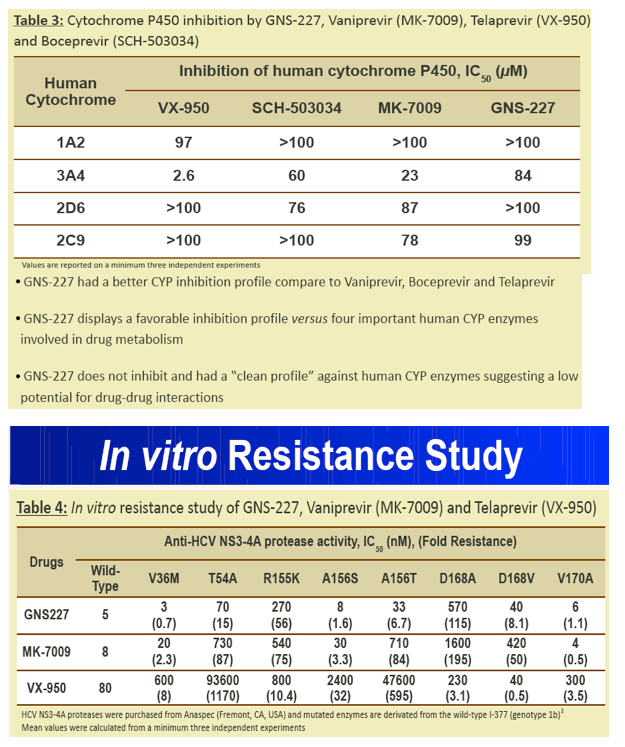
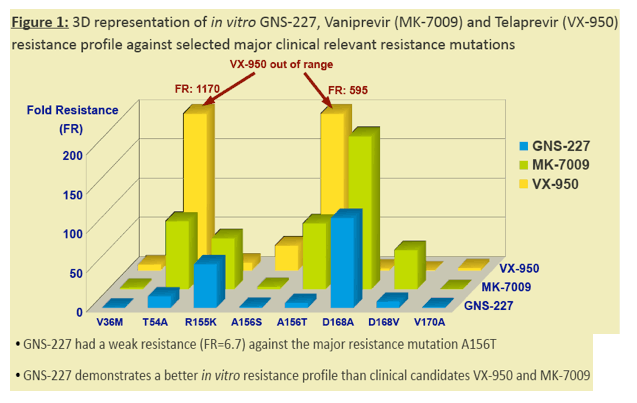
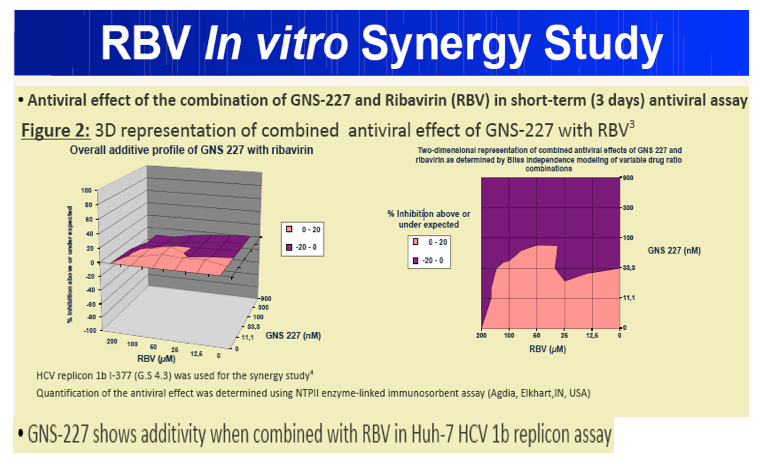
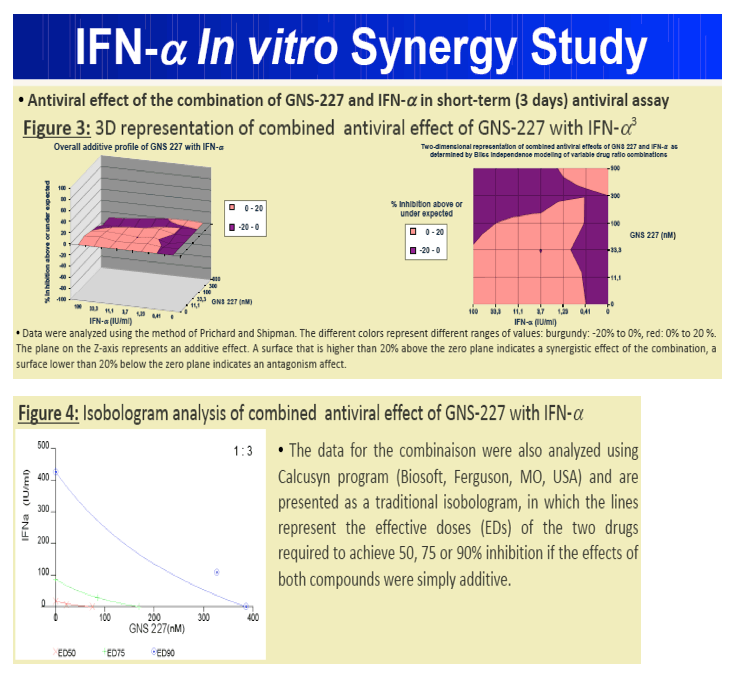
Results: GNS-227 demonstrated excellent potency in the low nanomolar range in an HCV replicon genotype 1b assay (EC50 = 9.2 nM) with no cytotoxicity up to 10 μM. GNS-227 was a potent and selective inhibitor of the NS3 protease with an IC50 of 5 nM against wild type and 0.003-0.57 μM against a panel of eight proteases containing various PI mutations. The fold resistance was low (<10 fold) against clinically relevant major resistance mutations (V36M, T54A, A156S, and A156T) when compared with other protease inhibitors (e.g., MK-7009, VX-950). GNS-227 showed several thousand-fold selectivity relative to a panel of human proteases (elastase, cathsepsin G/S, chymotrypsin, plasmin, thrombin, chymase: IC50 > 100 μM). Moreover, GNS227 did not show inhibition of cytochrome P450 (CYP1A2, 3A4, 2C9, 2D6: IC50 > 80 μM).
Conclusions: GNS-227 is a novel highly potent and specific HCV NS3 protease inhibitor in cellular and biochemical assays that has potential in preventing and treating emerging resistant variants. GNS-227 has favourable in vitro characteristics that warrant further pharmacological evaluation based on its potency, selectivity, activity against resistant mutants, lack of P450 enzyme inhibition and a wide therapeutic index.
References
1. (a) Halfon, P. et al. J. Hepatol. 2011 in press, (b) Courcambeck, J. et al. Antivir. Ther. 2006 (11), 847-855.
2. Taliani, M. et al. Anal. Biochem. 1996 (240), 60-67.
3. Prichard, M.N. and Shipman, C. Antiviral Res. 1990 (14), 181-206.
4. Lohmann, V. et al. Science 1999 (285), 110-113.
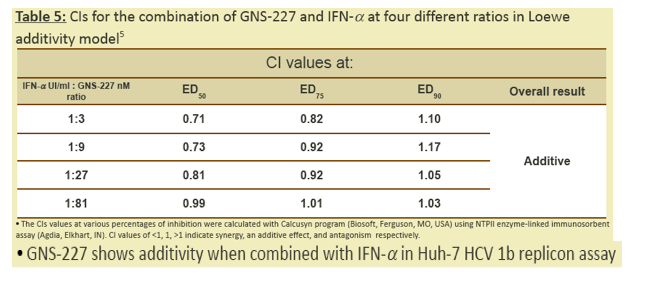
5. (a) Chou, T.C. and Talalay, P. Adv. Enzyme Regul. 1984 (22), 27-55, (b) Chang, T. T. and Chou, T.C. Acta Paediatr. Taiwan 2000 (41), 294-302.
|
| |
|
 |
 |
|
|