 |
 |
 |
| |
Safety and efficacy of etravirine in HIV-1-infected, treatment-experienced children and adolescents (6 to <18 years): Week 24 primary analysis of the Phase II PIANO study
|
| |
| |
Reported by Jules Levin
6th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Rome, Italy, 17-20 July 2011
Gareth Tudor-Williams,1 Pedro Cahn,2 Kulkanya Chokephaibulkit,3 Jan Fourie,4 Christos Karatzios,5 Stephanie Dincq,6 Thomas N Kakuda,7 Steven Nijs,6 Johan Vingerhoets,6 Frank Tomaka7
1Imperial College, London, UK; 2Fundacion Huesped, Buenos Aires, Argentina; 3Mahidol University, Bangkok, Thailand; 4Dr Jan Fourie Medical Practice, Dundee, KZN, South Africa; 5McGill University Health Centre, Montreal, Canada; 6Tibotec BVBA, Beerse, Belgium; 7Tibotec Inc., Titusville, USA
AUTHOR CONCLUSIONS
· Paediatric and especially adolescent populations remain difficult to treat given the known challenges with adherence and limited number of available antiretrovirals suitable for paediatric use.
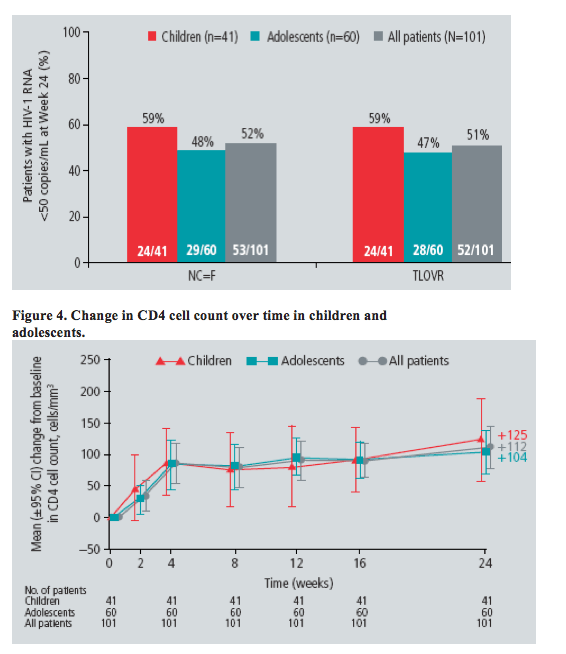
· The proportion of patients achieving viral load <50 HIV-1 RNA copies/mL at Week 24 was somewhat lower in adolescents versus children; the overall response rate of 52% was comparable to the response seen in adult patients in the DUET trials at Week 24.1,2
· Of those patients with virological failure, the most frequently emerging mutations have been previously described as etravirine resistance-associated mutations.
· Etravirine 5.2mg/kg bid (maximum dose 200mg bid) demonstrated safety and some efficacy in this treatment-experienced population of HIV-1-infected children and adolescents aged 6 to <18 years.
Introduction
· The efficacy and safety of the NNRTI etravirine has been demonstrated in the Phase III DUET trials in treatment-experienced, HIV-1-infected adult patients.1-4
· There is a need for additional antiretrovirals with appropriate formulations for use in paediatric patients.
· The short-term safety and pharmacokinetics of etravirine have been investigated in a Phase I trial (TMC125-C126)5,6
- an etravirine dose of 5.2mg/kg twice daily (bid), to a maximum dose of 200mg bid, was selected for further investigation in children and adolescents.
· PIANO (Pediatric trial with Intelence as an Active NNRTI Option; TMC125-C213; NCT00665847) is an ongoing 48-week, Phase II, open-label trial investigating the antiretroviral activity, safety and pharmacokinetics of etravirine 5.2mg/kg bid (maximum dose 200mg bid) in HIV-1-infected, treatment-experienced children and adolescents aged 6 to <18 years (Figure 1).
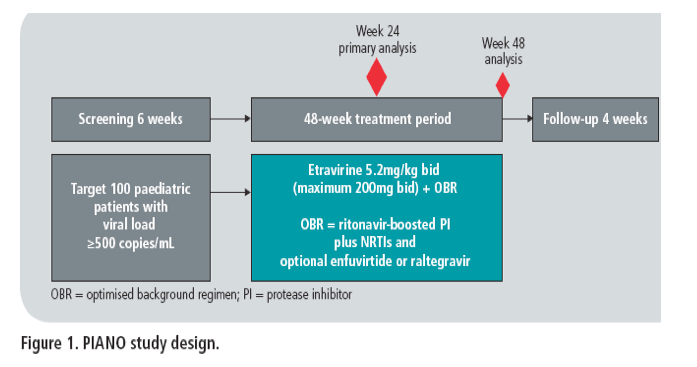
· The primary objective of the trial was to evaluate the safety and tolerability of etravirine in combination with other antiretrovirals over a 24-week treatment period in children and adolescents aged 6 to <18 years. Secondary objectives included
- longer-term safety and tolerability over 48 weeks
- antiviral activity of etravirine over a 24-week and a 48-week treatment period
- immunological changes (as measured by CD4 and CD8 cell count and CD4/CD8 ratio) over 24 and 48 weeks
- pharmacokinetic and pharmacodynamic parameters over 24 and 48 weeks.
· Safety and efficacy results from the Week 24 primary analysis are presented.
Methods
Study design
· Treatment-experienced, HIV-1-infected children (aged 6 to <12 years) and adolescents (aged 12 to <18 years) currently virologically failing on a stable regimen with confirmed viral load ≥500 HIV-1 RNA copies/mL received etravirine 5.2mg/kg bid (maximum 200mg bid) plus an investigator-selected OBR for 48 weeks (Figure 1)
- the OBR consisted of ritonavir-boosted atazanavir, darunavir, lopinavir or saquinavir in combination with N(t)RTIs, optional enfuvirtide and/or raltegravir; at least two active background antiretrovirals, based on screening vircoTYPE®, were mandatory
- there was a maximum screening period of 6 weeks.
· Written informed consent was obtained from all participants or their parent/legal guardian prior to any study-related procedure. Protocols were approved by the appropriate ethical committees and authorities, and the study was carried out in accordance with the principles of Good Clinical Practice, the Declaration of Helsinki and the European Union Clinical Trials Directive.
· Major exclusion criteria included any evidence of etravirine resistance (based on resistance testing at screening), presence of any currently active AIDS defining illness, and any grade 3 or 4 toxicity according to the DAIDS scale (except grade 3 absolute neutrophil count, grade 3 platelets, asymptomatic grade 3 pancreatic amylase, triglyceride, cholesterol or glucose elevation or grade 4 triglyceride elevation).
Safety analyses
· Safety is being monitored throughout the trial by an independent safety review panel.
· All adverse events (AEs) are followed until satisfactory clinical resolution; all grade 3 and 4 laboratory abnormalities as well as abnormalities resulting in an increase of two DAIDS grades from baseline are followed until return to baseline or within one grade from baseline.
Etravirine adherence
· Etravirine adherence is being measured by pill count and using selected questions from the Paediatric European Network for the Treatment of AIDS (PENTA) self-reported adherence questionnaire
- pill counts are recorded at each visit; questionnaires are completed at Weeks 2, 8, 16, 24, 32, 40 and 48.
Statistical analyses
· The primary analysis was performed when all patients had completed 24 weeks of treatment or had discontinued earlier.
· The primary population was the intent-to-treat population, defined as those patients who received at least one dose of study treatment, regardless of their compliance with the trial protocol.
· Analyses were performed by trial period (screening, treatment to Week 24, follow-up) and by age group (6 to <12 years and 12 to <18 years).
· The primary imputation for the calculation of virological response was the non-completer = failure (NC=F) method; the time to loss of virological response (TLOVR) rate was also calculated.
RESULTS
Patient demographics and baseline characteristics
· Of 178 screened patients from 48 sites, 101 were successfully screened and treated with etravirine
- sixty-three per cent of patients were female and 49% were White.
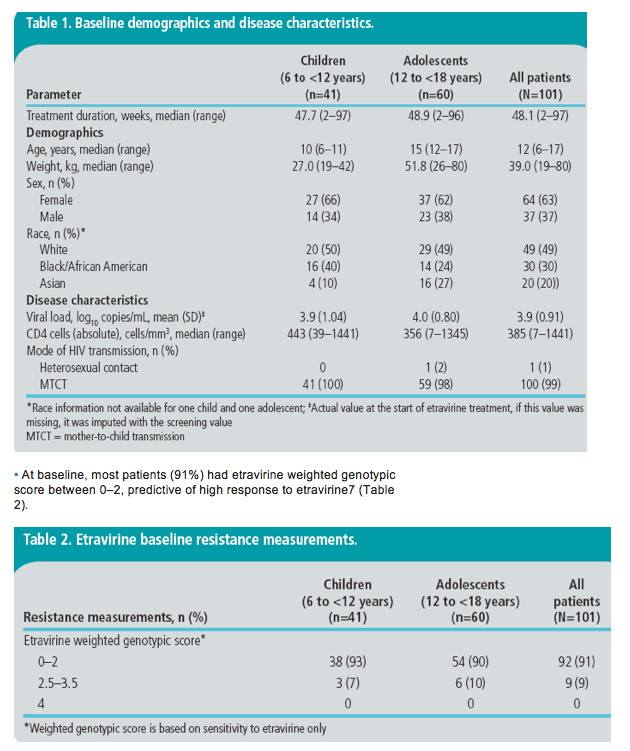
· Baseline characteristics by age group and overall are shown (Table 1).
· At baseline, most patients (91%) had etravirine weighted genotypic score between 0-2, predictive of high response to etravirine7 (Table 2).
Safety results
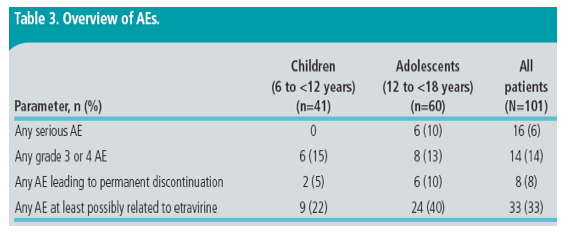
· Overall safety findings were generally similar between the age groups; the incidence of any serious AE, grade 3 or 4 AE, or AE leading to permanent discontinuation was low (Table 3)
- eight (8%) patients discontinued the trial due to an AE; of these, two patients discontinued due to grade 2 rash (one child and one adolescent) and two patients discontinued due to grade 3 rash (two adolescents)
- no fatal outcomes/events were reported
-- more adolescents than children experienced a serious AE, an AE leading to permanent discontinuation or an AE considered at least possibly related to etravirine treatment.
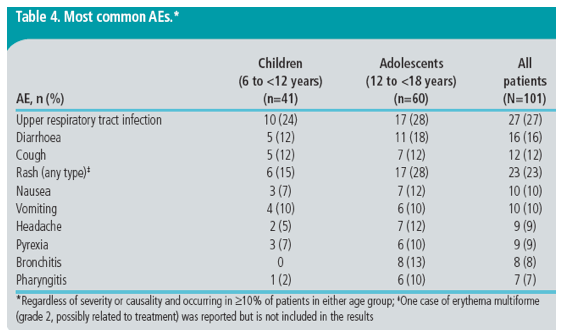
· The most common AEs, regardless of severity or causality, occurring in ≥10% of patients in either age group during etravirine treatment, are shown in Table 4.
· Rash, nervous system and psychiatric AEs were reported in 23%, 12% and 3% of patients, respectively
Drug relationship to AEs
· AEs of at least grade 2 severity considered at least possibly related to etravirine were recorded in 15% of children and 25% of adolescents; the overall incidence was 21%.
Laboratory parameters
· Most laboratory toxicities were grade 1 or 2 in severity.
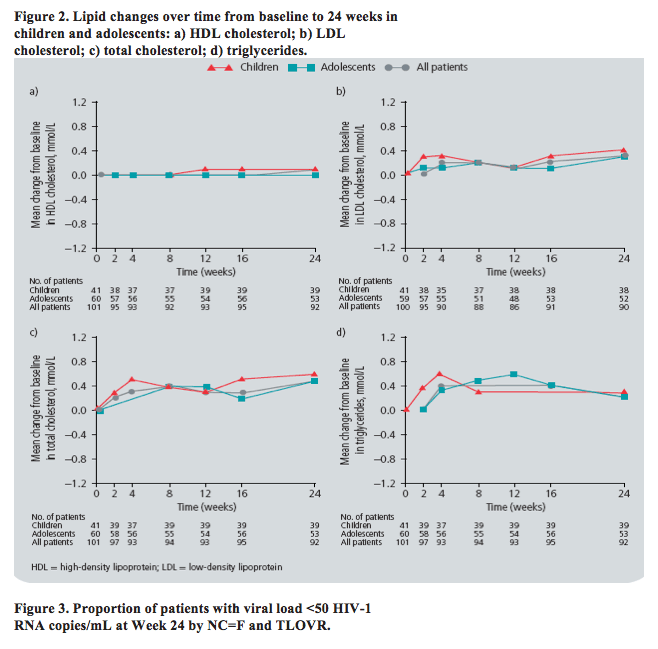
· Consistent with the known lipid profile of etravirine in adult patients, there were no consistent or clinically relevant changes in lipid parameters over time (Figure 2).
Resistance data
· In total, 43 patients (43%) were classified as virological failures (15 children and 28 adolescents)
- thirty-three patients were classified as non-responders and 10 as rebounders.
· Of 28 patients with available genotype at the time of virological failure (eight children and 20 adolescents), 15 (54%) (three children and 12 adolescents) developed NNRTI resistance-associated mutations, predominantly Y181C, E138A and V90I.
Adherence
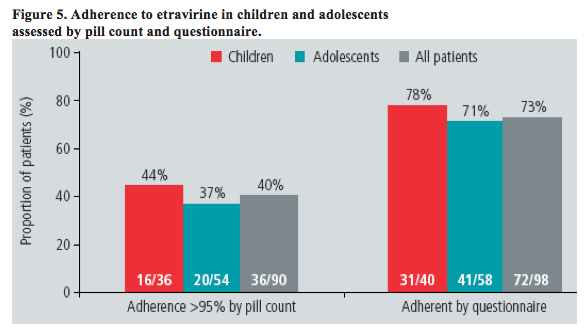
· Adherence to etravirine was measured by both pill count and questionnaire
- patients declaring not to have missed a single dose of etravirine on the 3 days prior to filling out the questionnaire were considered to be adherent for that visit. However, once a patient was declared to be non-adherent (at least one missed dose of etravirine during the last 3 days), they were classified as non-adherent at all subsequent visits
- etravirine adherence by pill count and questionnaire are shown in Figure 5
-- etravirine adherence by self-reported questionnaire was notably higher than when estimated by pill count.
· Virological response rates (<50 HIV-1 RNA copies/mL) were analysed by etravirine adherence, as judged by pill counts and by questionnaire (Figure 6).
References
1. Madruga J, et al. Lancet 2007;370:29-38.
2. Lazzarin A, et al. Lancet 2007;370:39-48.
3. Katlama C, et al. AIDS 2009;23:2289-300.
4. Katlama C, et al. AIDS 2007;21:395-402.
5. Kakuda TN, et al. CROI 2008. Abstract 578.
6. Konigs C, et al. CROI 2009. Abstract 879.
7. Vingerhoets J, et al. AIDS 2010;24:503-14.
8. Katlama C, et al. EACS 2007. Abstract P7.3/11.
|
| |
|
 |
 |
|
|