 |
 |
 |
| |
Safety and pharmacokinetics (PK) of single rising oral doses of a novel HIV integrase inhibitor in healthy volunteers
|
| |
| |
Reported by Jules Levin
ICAAC Sept 17-20 2011 Chicago
S. Aslanyan1, Ch. Ballow3, J. P. Sabo1, J. Habeck2, D. Roos1, T. R. MacGregor1, P. Robinson1, J. Kort1
1 Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, US 2 Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany 3 Buffalo Clinical Research Center, Buffalo, NY, US
ABSTRACT
Background: BI 224436 is a novel non-catalytic site HIV integrase inhibitor.
Methods: Double-blind, placebo-controlled, first-in-human, single rising dose trial of an oral solution formulation of BI 224436 in healthy male volunteers. Forty-eight subjects were randomized to 6 ascending consecutive dose cohorts (BI 224436 6.2, 12.5, 25, 50, 100 and 200 mg); 6 subjects received BI 224436 and 2 received placebo within each cohort.
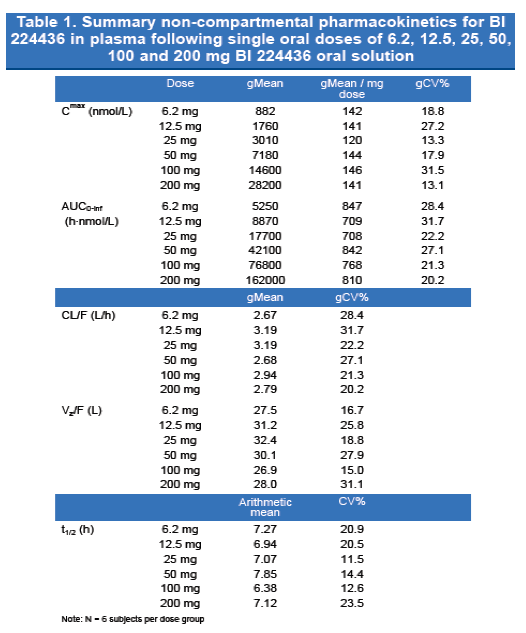
Results: Geometric means (gmean) of selected PK parameters and dose cohorts are presented in the table.

Dose-proportional increases in plasma BI 224436 Cmax, AUC0-∞, AUC0-24, and AUC0-tz were observed. Inter-subject variability (geometric CV%) was < 33% for these PK parameters. Oral solution of BI 224436 was rapidly absorbed with a median Tmax of 0.5 h (range, 0.25-1.5 h). Arithmetic mean terminal elimination half-life (t1/2) was 7.11 h and consistent across all groups and variability (arithmetic CV%) was < 24%.
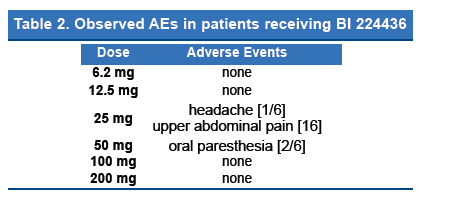
Four mild adverse events in 4 subjects were observed within 72 h of administration of BI 224436 without any dose related trend (headache [1] and upper abdominal pain [1] in 25 mg cohort and oral paresthesia [2] in 50 mg cohort). No clinically relevant changes in vital signs and ECG and laboratory parameters were noted.
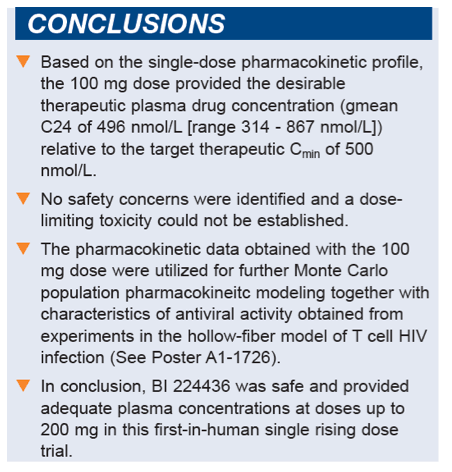
Conclusion: Single doses of BI 224436 were safe and well-tolerated up to the highest dose tested. Based on the single-dose PK profile, the 100 mg dose provided the desirable therapeutic plasma drug concentration at 24 h after dosing (gmean 496 nmol/L [range 314 - 867 nmol/L]) relative to a targeted therapeutic Cmin of 500 nmol/L. These results indicate that once-daily dosing with BI 224436 may be feasible.
INTRODUCTION
BI 224436, a novel allosteric (non-catalytic) site HIV-1 integrase inhibitor, provides an opportunity to further exploit inhibition of the HIV integrase enzyme by a mechanism that is distinct and complementary to contemporary integrase strand transfer inhibitors.
- BI 224436 exerts this inhibition by binding to a highly conserved allosteric pocket on the catalytic core of integrase which also serves as binding pocket for LEDGF, a host cell co-factor important for viral replication (See Poster F1-1369).
- Based on preclinical pharmacology data for BI 224436 (see Posters F1-1369 and A1-1726), a Cmin in humans of approximately 500 nM has been selected.
- Significant differences in the pharmacokinetics (PK) of BI 224436 among the various animal species tested were observed, which made it not feasible to predict PK in humans, hence this first-in-human trial was advanced.
METHODS
- Single rising dose, randomized, double-blind, and placebo controlled within dose groups.
- First-in-human study conducted in healthy adult male volunteers at a single center.
- Tested sub-therapeutic doses and escalated up to doses in the therapeutic range.
- Dose escalation from each dose cohort to the next higher dose cohort was based on the assessment of safety and PK.
- The trial was conducted according to ICH GCP and was approved by research center IRB. All subjects have signed and dated written informed consent prior to admission to the study.
- The primary objective of the study was to obtain single-dose PK of BI 224436 in healthy volunteers that may predict whether BI 224436 could be administered once daily. Another objective was to obtain first data on the safety profile of the compound.
Bioanalytical
Concentrations of BI 224436 were determined in EDTA plasma by a validated HPLC-MS/MS assay (high performance liquid chromatography, tandem mass spectrometry) with a lower limit of quantification (LLOQ) of 5.00 nmol/L.
PK end-points for BI 224436 were determined from the plasma BI 224436 concentration-time profiles using non-compartmental methods (WinNonlin Professional v5.3; Pharsight Corporation, Sunnyvale, CA) (Table 1).
Safety was assessed descriptively based on:
- Physical examination
- Vital signs (BP, PR, RR, oral body temperature)
- 12-lead ECG and telemetry
- Clinical laboratory tests (hematology, clinical chemistry and urinalysis)
- Adverse events
Subject Eligibility Criteria:
1. Healthy males
2. Age ≥ 21 and ≤ 50 years
3. Body Mass Index (BMI) ≥ 18.5 and BMI ≤ 29.9 kg/m2
RESULTS
Forty-eight subjects were randomized in this trial with 8 subjects within each of 6 dose cohorts (6.2, 12.5, 25, 50, 100 and 200 mg). Within each dose cohort, 6 subjects received BI 224436 and 2 subjects received placebo, resulting in total 12 subjects receiving placebo. All subjects who were randomized received the assigned study drug and completed all trial visits.
The demographic data and baseline characteristics of the subjects entered into this study (all male) were reasonably similar in all cohorts. There were 28 White subjects, 17 African Americans and 3 Asians included. The overall median age (range) was 26.0 (21-50) years. The median BMI (range) was 25.35 (18.9-29.8) kg/m2.
Pharmacokinetics:
- A summary of selected PK parameters following single-dose BI 224436 administration is presented in Table 1.
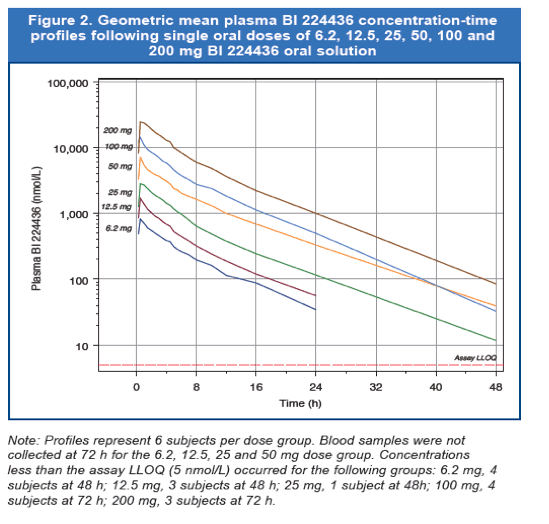
- Plasma BI 224436 Cmax, AUC0-∞, AUC0-24, and AUC0-tz increased in a dose proportional manner over the dose range of 6.2 to 200 mg (Figure 2).
- Variability (geometric CV%) was low and less than 32% for these PK parameters (gCV% range: Cmax, 13.1 - 31.5%; AUC0-∞, 20.2 - 31.7%).
- BI 224436 was rapidly absorbed with Tmax occurring quickly after oral administration (median 0.5 h in each dose group; overall range 0.25 - 1.5 h).
- Arithmetic mean terminal elimination half-life (t1/2) was consistent across the 6 dose groups and ranged from 6.38 h to 7.85 h (arithmetic mean 7.11 h); variability (arithmetic CV%) was low and less than 24% (range 11.5 - 23.5%).
- Based on the single-dose pharmacokinetic profile, the 100 mg dose provided the desirable therapeutic plasma drug concentration (geometric mean C24 of 496 nmol/L [range 314 - 867 nmol/L]) relative to the target therapeutic Cmin of 500 nmol/L.
Safety:
- There were no SAEs during the trial.
- Only 4 mild AEs were observed (Table 2).
- Real-time 3-lead ECG telemetry monitoring and intensive point 12-lead ECG monitoring did not identify any safety concerns.
- No clinically relevant changes in vital signs and laboratory parameters were observed.
REFERENCEs
Poster F1-1369: Pre-Clinical profile of BI 224436, a Novel HIV-1 Non-Catalytic Site Integrase Inhibitor. C. Fenwick, M. D. Bailey, R. Bethell, P. Bonneau, M. Cordingley, R. Coulombe, J. Duan, P. Edwards, L. Fader, M. Garneau, A. Jakalian, S. Kawai, S. Laplante, L. Luo, S. Mason, M-A Poupart , B. Simoneau, Y. Tsantrizos, and C. Yoakim. ICAAC 2011, Chicago.
Poster A1-1726: Pharmacodynamics of BI 224436 for HIV in an In Vitro Hollow Fiber Infection Model System. A. Brown, J. Mcsharry, R. Kulawy, C. Fenwick, R. Bethell, J. Duan, J. Kort, P. Robinson, B. Simoneau, C. Yoakim, and G. Drusano. ICAAC 2011, Chicago.
|
| |
|
 |
 |
|
|