 |
 |
 |
| |
Pharmacokinetic analysis of nevirapine extended release 400 mg QD vs immediate release 200 mg BID
in patients with HIV-1 infection
|
| |
| |
Reported by Jules Levin, 51st ICAAC Chicago Sept 17-20 2011
FDA approves one-pill, once-daily Viramune® XR (nevirapine) extended-release tablets for use in combination with other antiretroviral agents for treatment of HIV-1 infection in adults - (04/07/11)
Once-Daily Nevirapine Noninferior to Twice-Daily Dose in Treatment Naive - (09/16/10)
C-L. Yong1, J. Gathe2, J. Rockstroh3, C. Orrell4, J. Morales5, J. Bogner6, T. Nguyen1, W. Zhang1, M. Drulak1, A-M. Quinson1
1Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT, USA; 2Therapauetic Concepts, Houston, TX, USA; 3Universitäts Klinik, Bonn, Germany; 4Desmond Tutu HIV Foundation, Cape Town, South Africa; 5Clin Res PR, San Juan, Puerto Rico; 6University Hospital Munich, Munich, Germany.
Abstract
Background: Week (Wk) 48 VERxVE study data showed non-inferiority (NI) in virologic efficacy of the nevirapine extended release 400 mg QD (NVP XR) formulation compared with NVP immediate release 200 mg BID (NVP IR). Steady-state pharmacokinetic (PK) results to Wk 48 are presented.
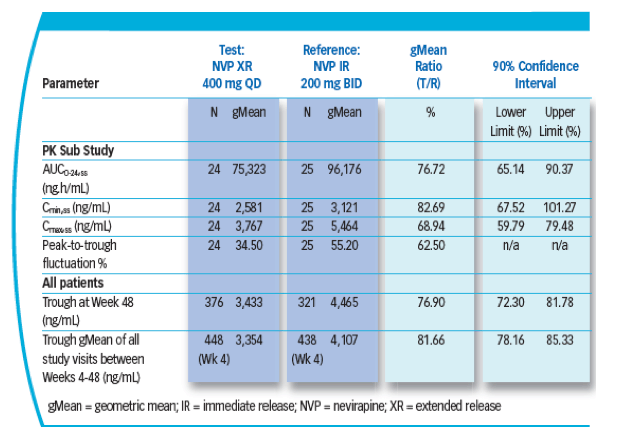
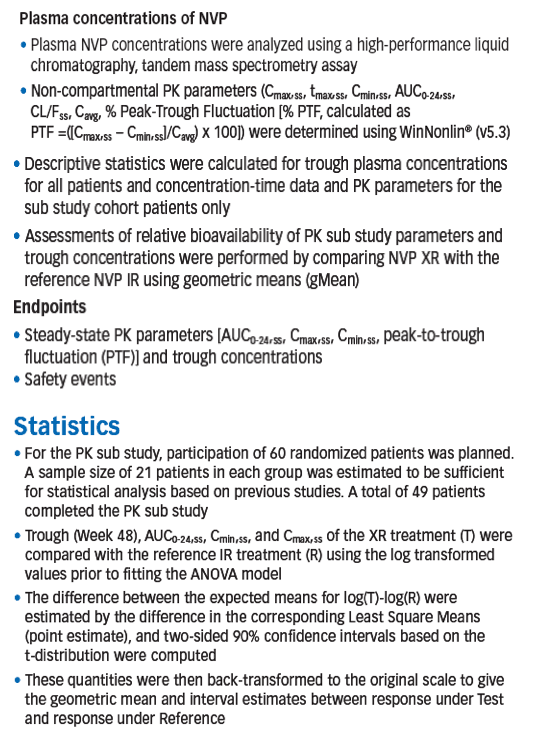
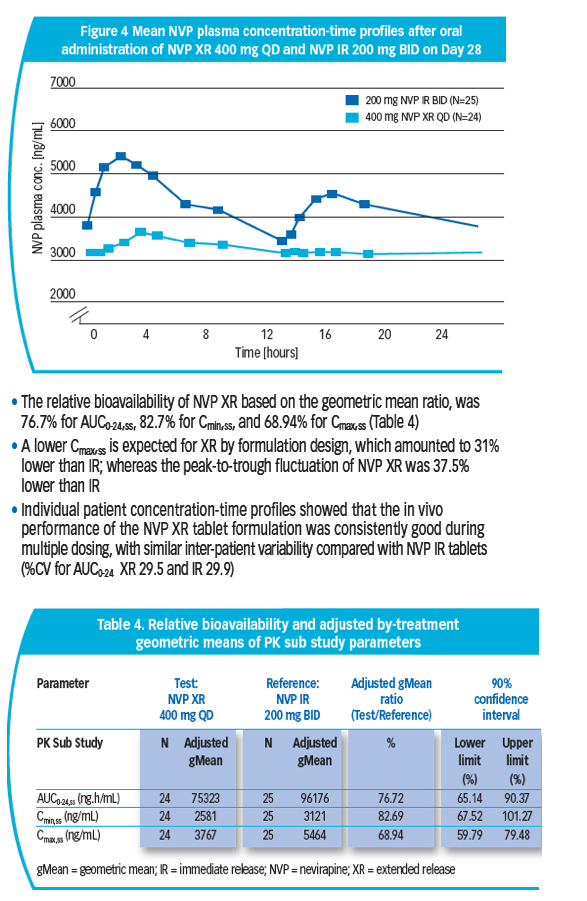
Methods: Double-blind, double-dummy NI study in treatment-naive adult patients with HIV-1, viral load >1000 copies/mL and CD4 count <400 cells/mm3 (males) and <250 cells/mm3 (females). Patients randomized to NVP XR (Test) or NVP IR (Reference). All randomized patients qualified for the opt-in PK sub study with 50 patients planned at Wk 4. 14 study centres with PK experience were eligible. Parameters for the PK sub study (AUC0-24,ss, Cmax,ss, Cmin,ss,
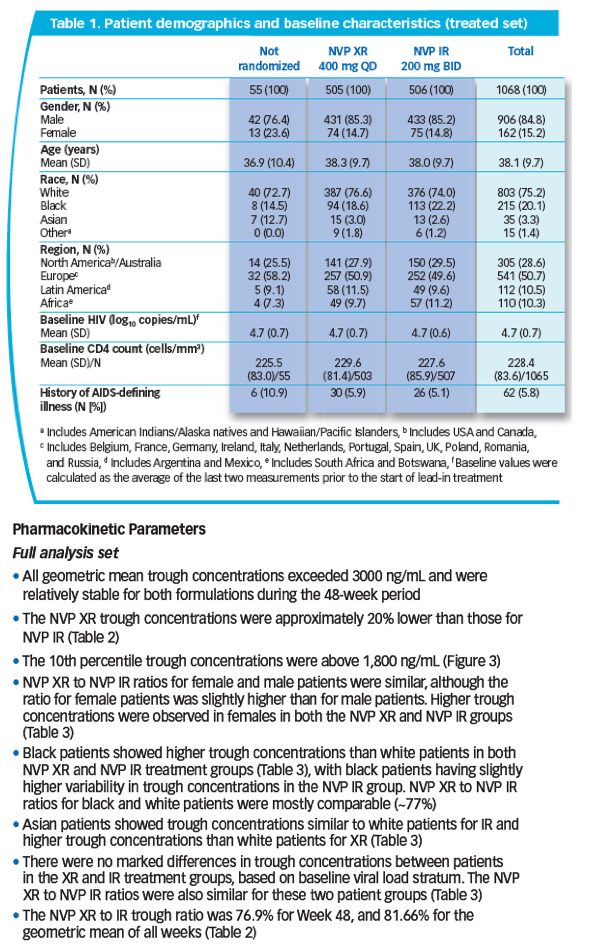
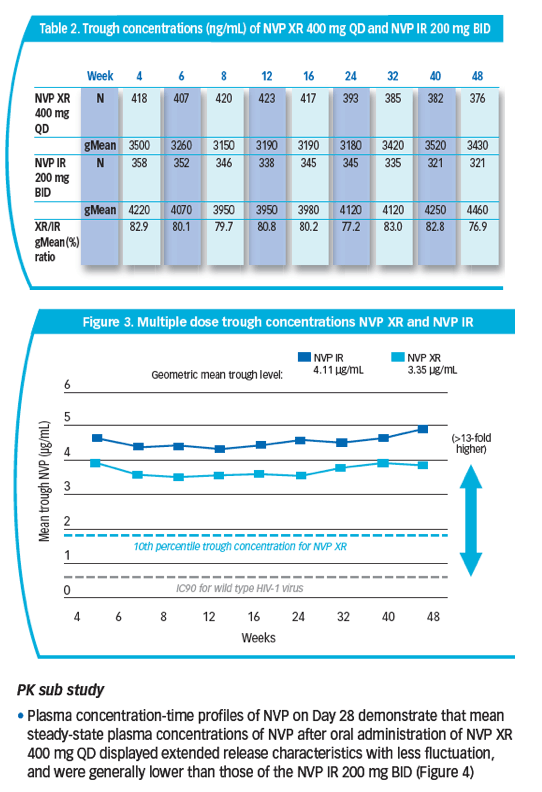
peak-to-trough fluctuation [PTF]) were obtained and relative bioavailability assessed at Wk 4 by plasma collection over 24 hrs. NVP trough concentrations for all entered patients were measured at Wk 48 and the geometric mean (gMean) of all study visits to Wk 48 were calculated. NVP concentrations were determined using LC tandem mass spectrometry.
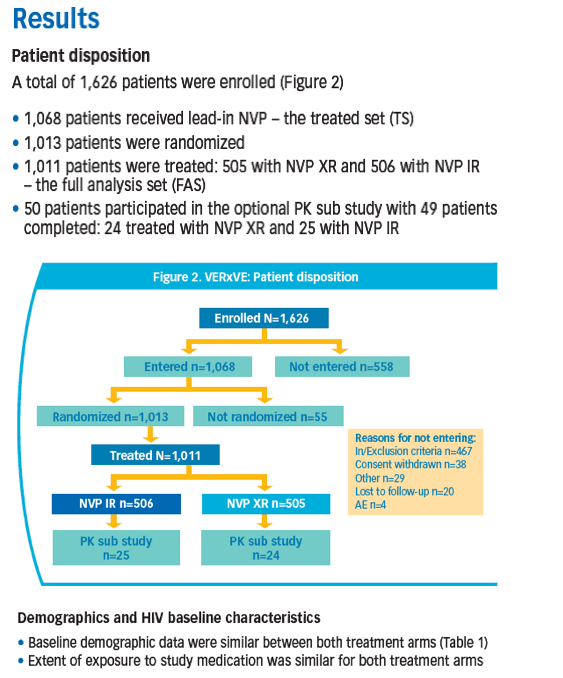
Results: 49/1011 pts from 14/215 sites entered the PK sub study: 24 received NVP XR, 25 NVP IR. NVP XR had less PTF (34.5%) than IR (55.2%). NVP XR exhibited lower PK parameters and trough concentrations than IR.
Conclusions: NVP XR 400 mg QD exhibited XR characteristics with less NVP PTF
than NVP IR. Trough levels over 48 wks were stable for both formulations with
NVP XR/IR ratios 77-82%. These data (with efficacy results) suggest NVP XR
compared with NVP IR achieved lower but effective NVP exposure.



|
| |
|
 |
 |
|
|