 |
 |
 |
| |
PPI-668, A Potent New Pan-Genotypic HCV NS5A Inhibitor: Phase 1 Efficacy and Safety
|
| |
| |
Reported by Jules Levin
AASLD
Nov 9-13 2012 Boston
J. Lalezari1, G. Farrell2, P. Shah3, E. Lawitz4, C. Schwabe5, D. Walsh1, P. Vig6, N. Brown6, E. Ruby6, S. Halfon6, N. Huang6, Q. Huang6, R. Colonno6, L. Li6, B. Johnston7, B. Wargin8 and E. Gane5 1Quest Clinical Research and UCSF, San Francisco, CA, USA; 2Canberra Hospital, Canberra, Australia; 3West Coast Clinical Trials, Costa Mesa, CA, USA; 4Alamo Medical Research, San Antonio, TX, USA; 5Auckland Clinical Studies, Auckland, New Zealand; 6Presidio Pharmaceuticals, San Francisco, CA, USA; 7CTI Clinical Trial and Consulting Services, Cincinnati, OH, USA; and 8PK-PM Associates, Chapel Hill, NC, USA
ABSTRACT
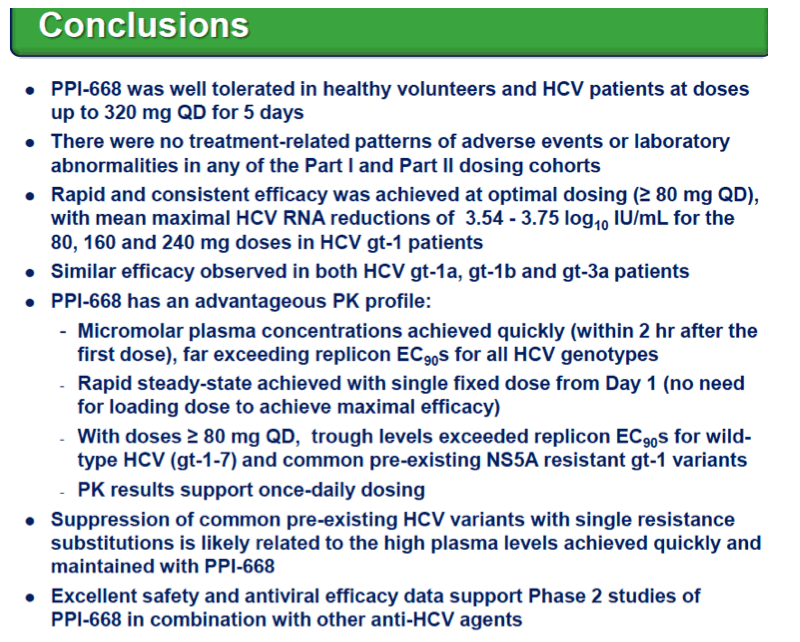
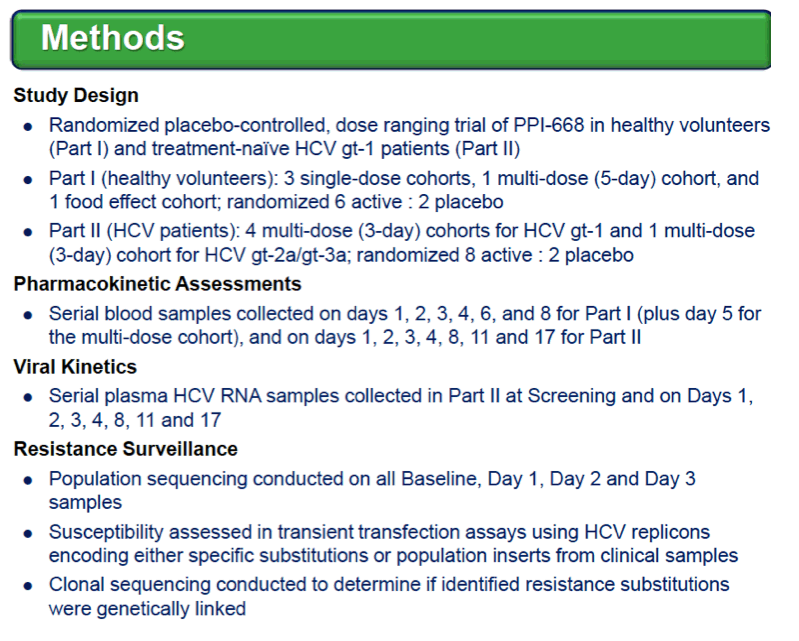
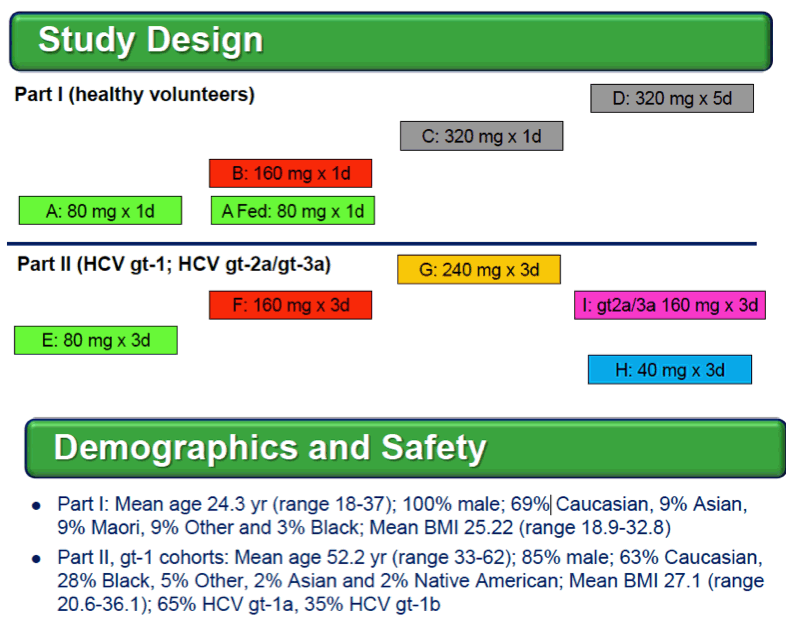
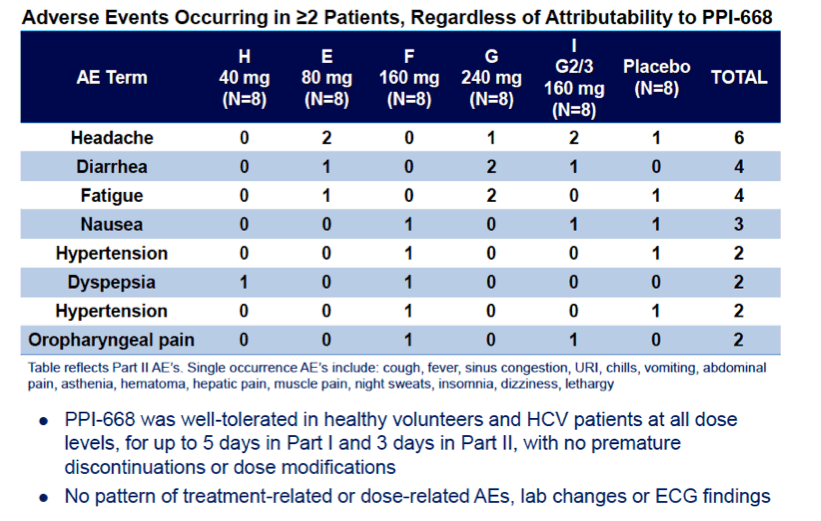
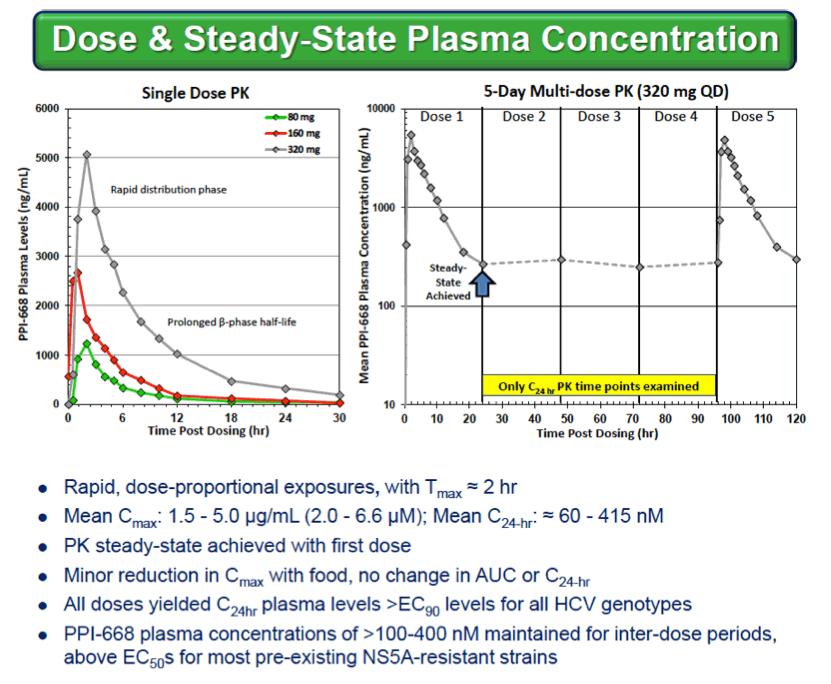
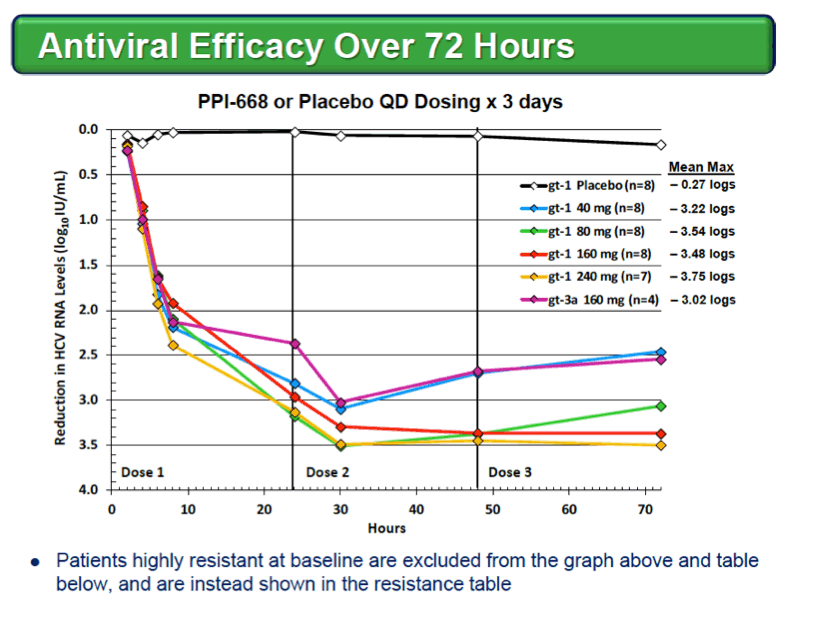
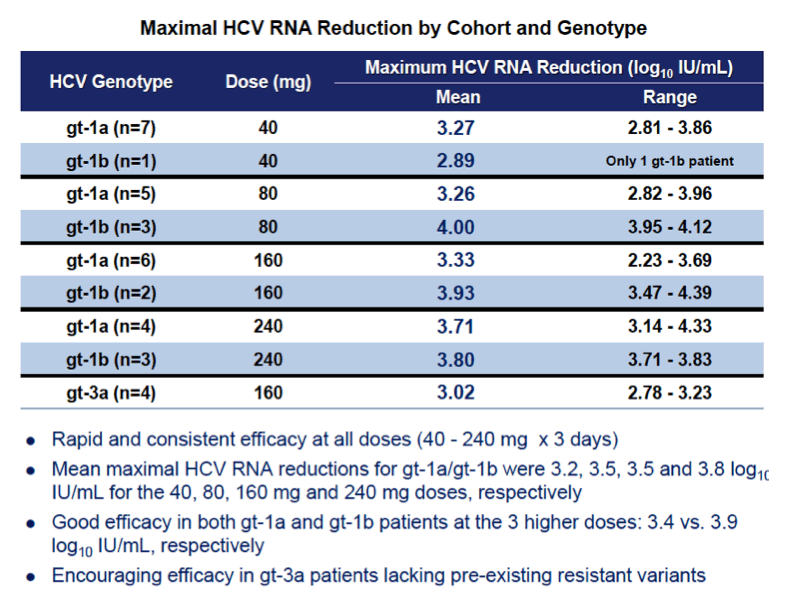
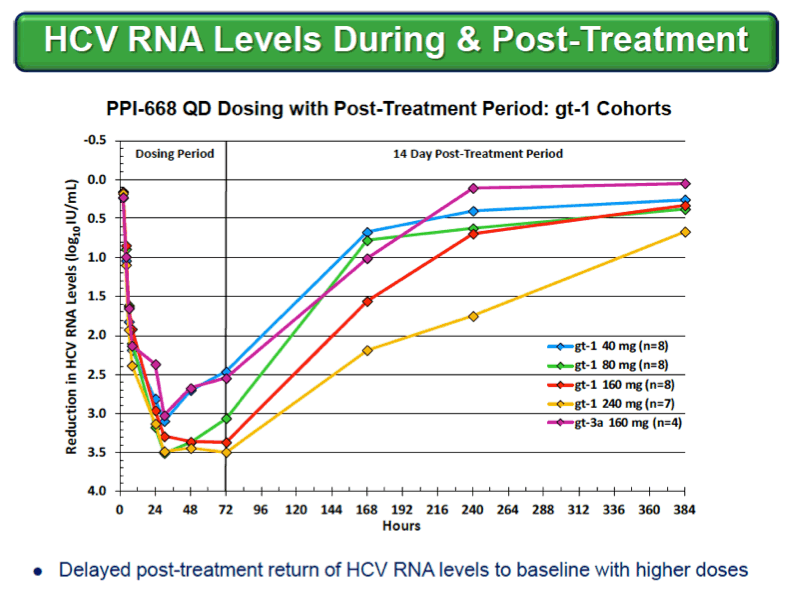
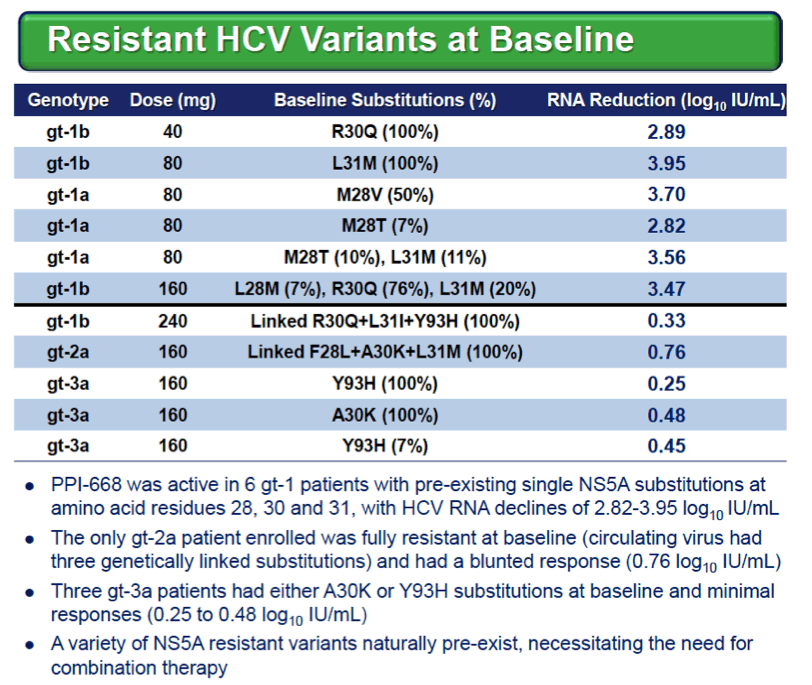
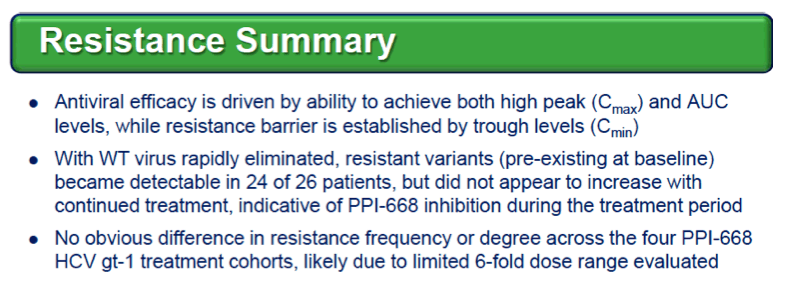
Background: Optimized HCV NS5A inhibitors can contribute potent pan-genotypic efficacy to HCV combination therapies. Here we report the preliminary safety and efficacy of PPI-668, a new NS5A inhibitor with 50% inhibitory concentrations (EC50s) of 0.02-1.3 nM in replicon assays for HCV genotypes 1-7 (gt1-gt7). Methods: This Phase 1 trial first assessed the safety and pharmacokinetics (PK) of PPI-668 in healthy volunteers (Part I). Single oral doses of 80,160 and 320 mg were sequentially evaluated with 8 volunteers per dose cohort randomized 6:2 to active PPI-668 capsules vs. placebo. A fourth cohort evaluated 320 mg QD for 5 days. Following completion of Part I, doses of 40, 80, 160 and 240 mg QD x 3 days were evaluated in treatment-na´ve patients with HCV-gt1 infection (Part II), with 10 patients per cohort randomized 8:2 (active vs. placebo). A cohort of patients with HCV gt2a or gt3a infection was subsequently added to explore the pan-genotypic clinical efficacy of PPI-668. Study evaluations included clinical and laboratory safety monitoring, PK, serum HCV RNA (by TaqMan™ PCR v2.0), and viral resistance monitoring. Results: PPI-668 was consistently well-tolerated at all dose-levels in both the 32 Part I volunteers and the 40 Part II HCV patients, with no serious or severe adverse events and no pattern of treatment-related (drug vs. placebo) or dose-related adverse effects. PK results in Part I indicated rapid absorption and dose-proportional plasma concentrations of PPI-668 that achieved μM levels, with 24 hr post-dose plasma concentrations remaining significantly above the PPI-668 EC50s for HCV gt1-gt7. Importantly, PPI-668 inter-dose plasma levels exceeded the EC90s for pre-existing resistant HCV gt1 variants encoding single NS5A resistance substitutions. Of the 32 active-dosed HCV-gt1 patients in Part II, 31 achieved rapid (1- to 3-day) multi-log virologic responses, with mean maximal HCV RNA reductions of 3.22 log10, 3.54 log10, 3.48 log10, and 3.75 log10 IU/mL for the 40, 80, 160 and 240 mg/day dose groups, respectively. One patient in the 240 mg group had a negligible HCV RNA reduction (0.23 log10 IU/mL); this patient was fully-resistant at baseline, with 100% of circulating virus encoding 3 genetically linked NS5A resistance substitutions. Conclusions: PPI-668 (40-320 mg) has been well-tolerated in volunteers and patients, with a PK profile supporting QD dosing. PPI-668 treatment produced rapid HCV RNA reductions of 3.54 - 3.75 log10 IU/mL in HCV-gt1 patients with the 3 higher doses. Results for the gt2-gt3 cohort produced mean maximal responses of 3.02 log10 with the 160 mg dose.

|
| |
|
 |
 |
|
|