 |
 |
 |
| |
Nucleos(t)ide analogues can be safely discontinued in CHB patients achieving HBsAg clearance
|
| |
| |
Reported by Jules Levin
AASLD Nov 9-13 2012 Boston
F.Invernizzi1, P.Lampertico1, A.Loglio1, M.Iavarone1, M.Vigan˛2, F.Facchetti1, E.Vezali1, G.Lunghi3, M.Colombo1.
1 1st Division of Gastroenterology, Fondazione IRCCS CÓ Granda Ospedale Maggiore Policlinico, UniversitÓ degli Studi di Milano, Milan, Italy.2 Liver Unit, Ospedale San Giuseppe, UniversitÓ degli Studi di Milano, Milan, Italy3 Institute of Preventive Medicine, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, UniversitÓ degli Studi di Milano, Milan, Italy.
CONCLUSIONS
In patients clearing HBsAg during NUC therapy, treatment can be safely discontinued following either anti-HBs seroconversion or at least 12 months of consolidation after HBsAg clearance
BACKGROUND & AIM
Though international guidelines recommend nucleos(t)ide analogues (NUC) to be stopped in chronic hepatitis B (CHB) patients achieving HBsAg seroconversion, whether this rule can be applied safely in clinical practice and for patients clearing HBsAg without seroconversion to anti-HBs, is unknown. Aim: To assess the outcome of patients achieving HBsAg clearance on NUC therapy.
Patients & Methods
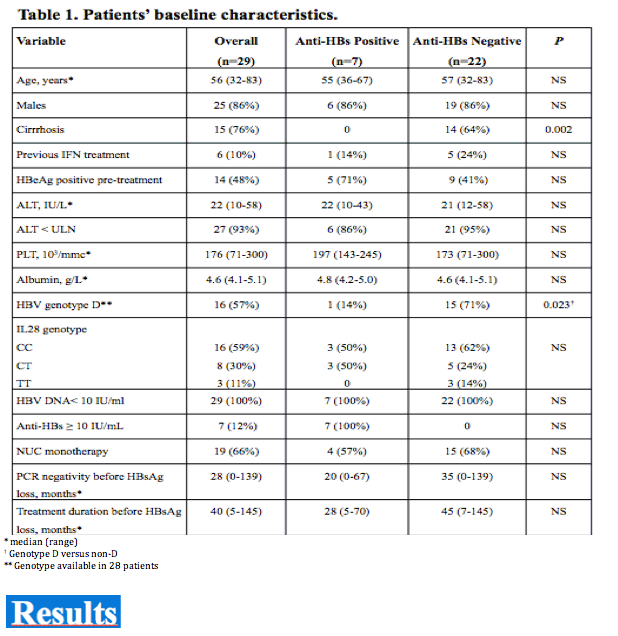
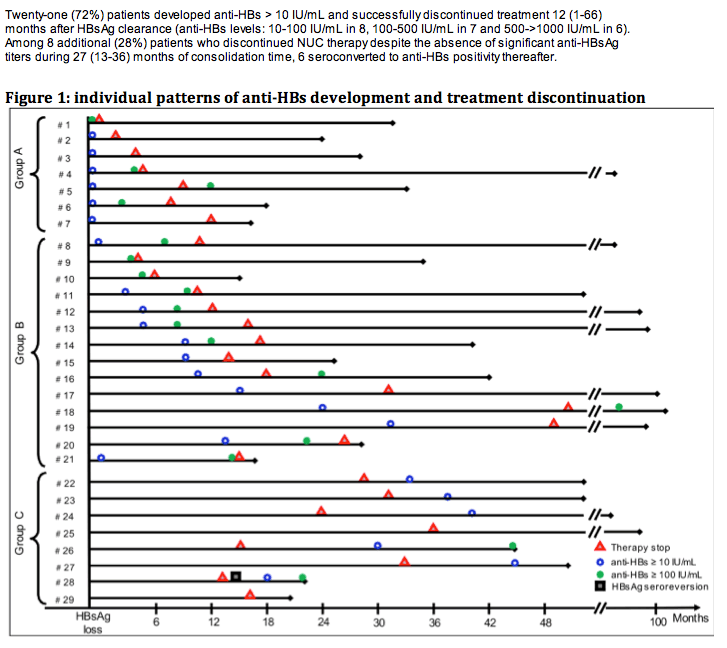
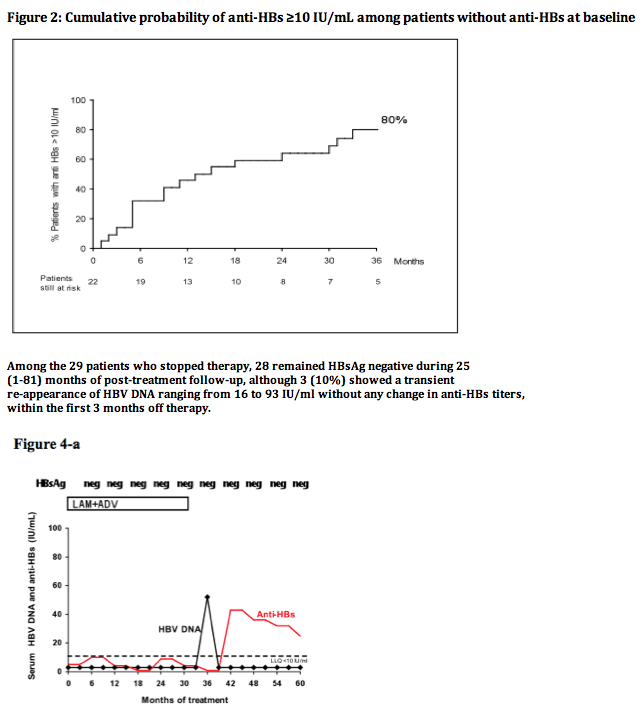
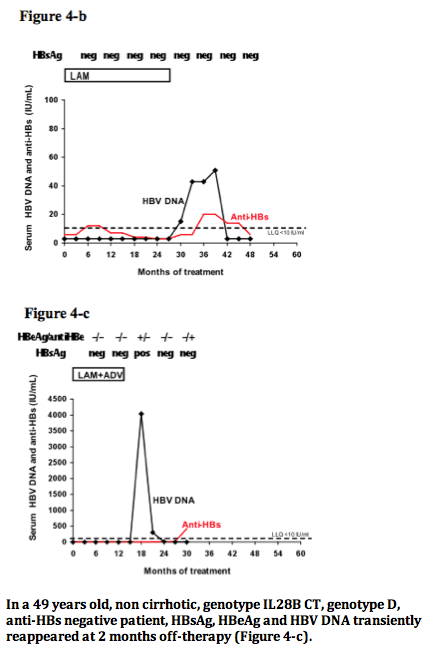
Among 520 CHB patients who received NUC between 1997-2008, 29 (6%) lost HBsAg and were followed for 42 (14-117) months thereafter. At the time of HBsAg clearance all patients had normal ALT, were HBeAg negative (52% HBeAg positive pre-treatment) and had undetectable HBV DNA (real time PCR < 10 IU/mL); median age was 56 years, 86% male, 52% cirrhotics, 55% genotype D, 55% CC genotype IL28B (SNP rs12979860). Nineteen (66%) patients received monotherapy (11 LAM, 3 ETV, 5 TDF) and 10 (34%) combination (5 LAM+ADV, 5 LAM+TDF); 6 (21%) were previously treated with interferon. HBsAg was quantified by Architect (Abbot Diagnostics, detection limit 0.05 IU/mL).
|
| |
|
 |
 |
|
|