 |
 |
 |
| |
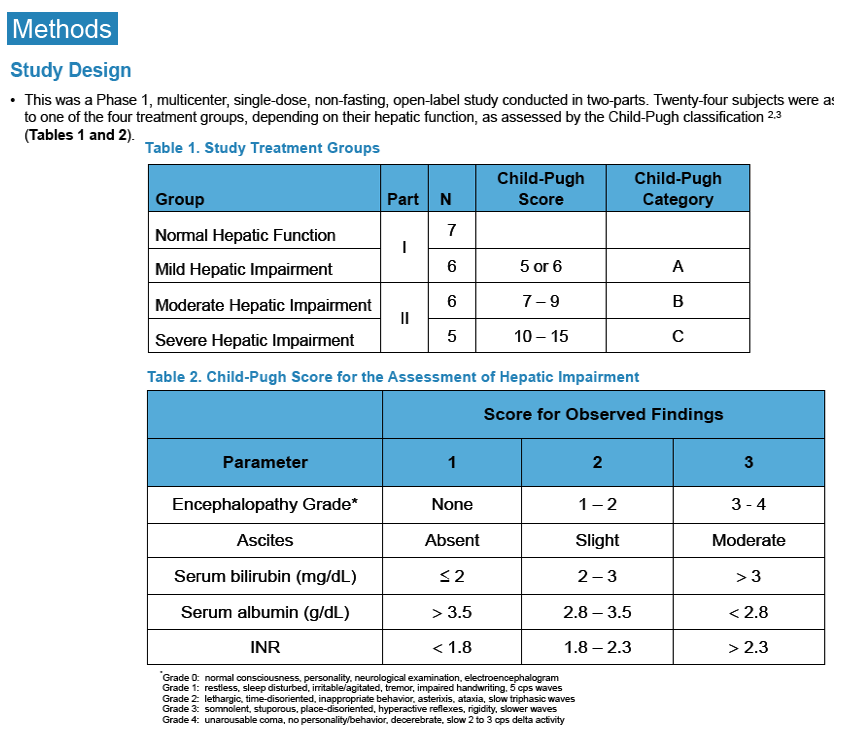
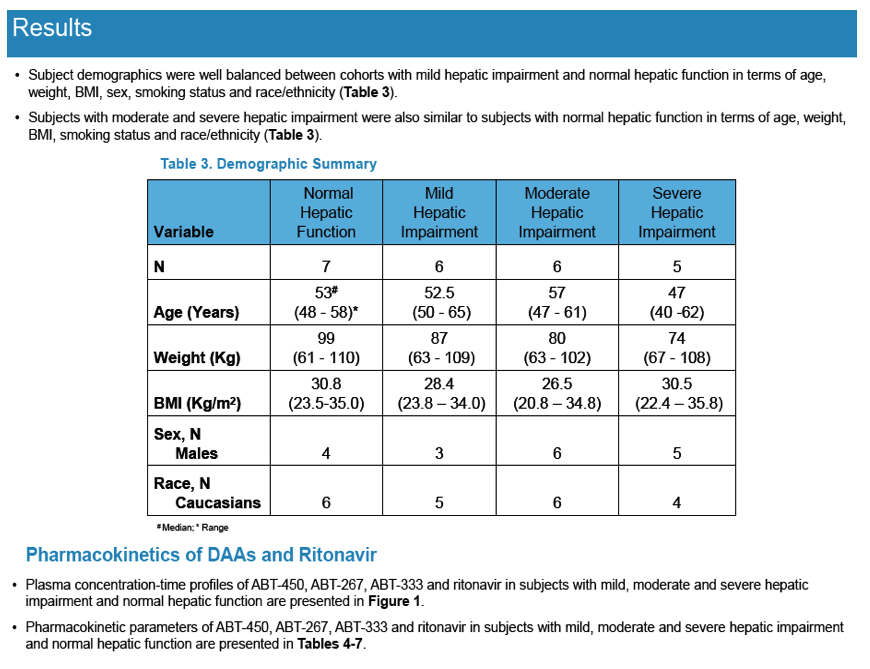
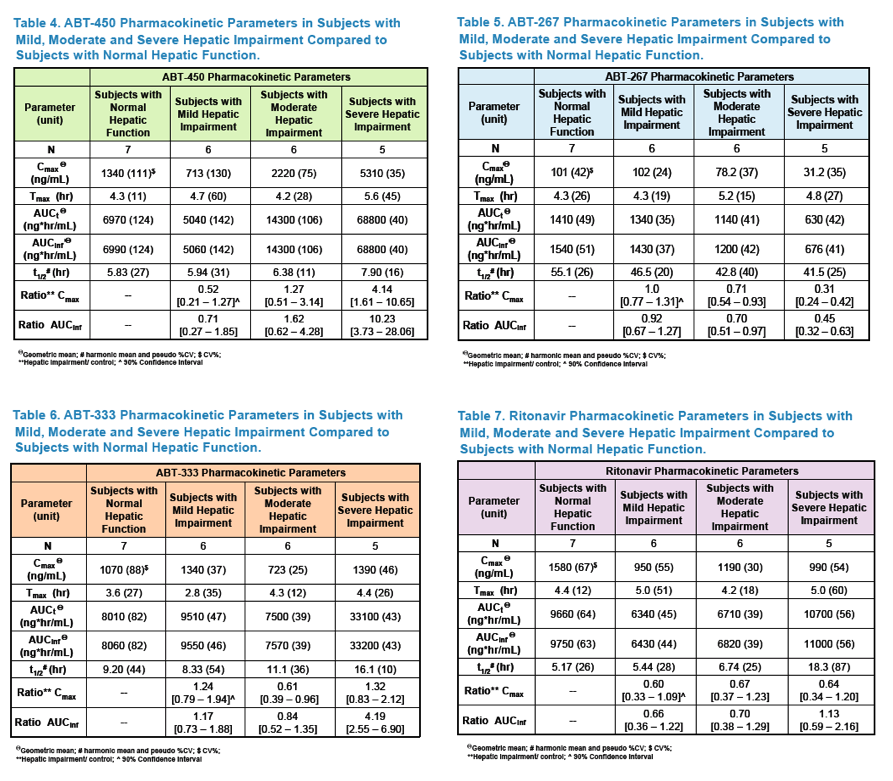
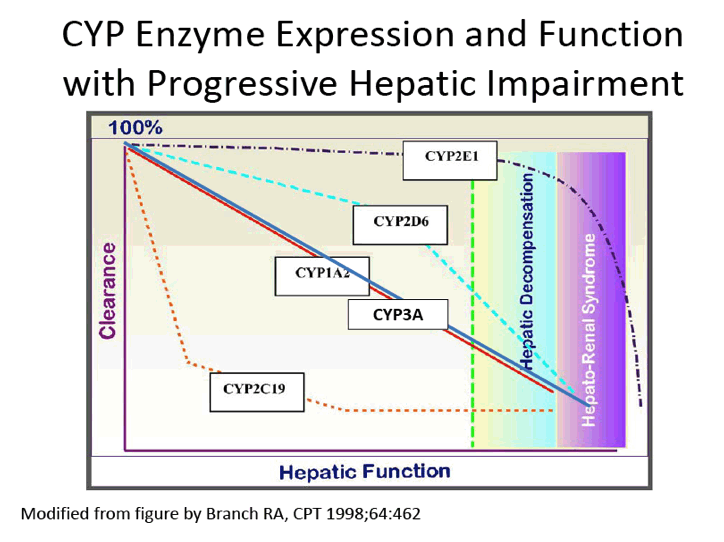
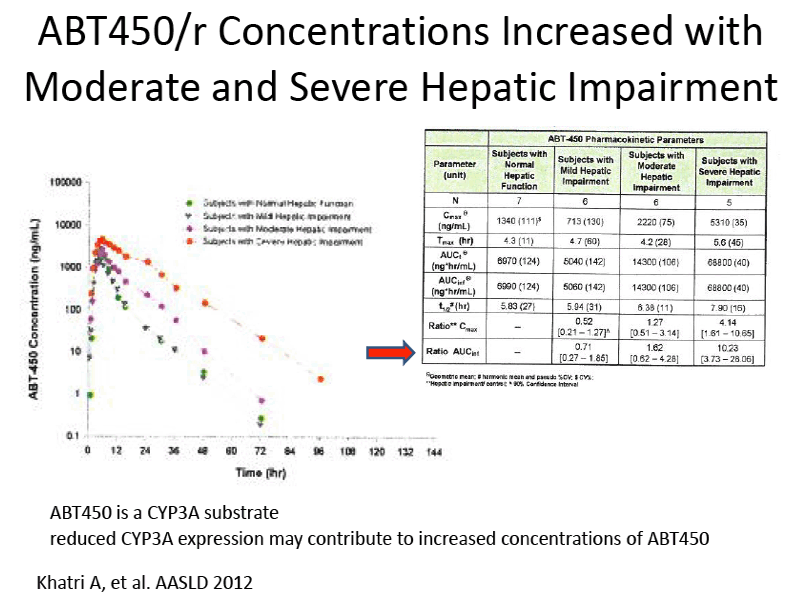
Pharmacokinetics and Safety of Co-administered ABT-450 plus Ritonavir (ABT 450/r), ABT-267 and ABT-333 as a Single Dose in Subjects with Normal Hepatic Function and in Subjects with Mild, Moderate and Severe Hepatic Impairment (DAAs PK; Sofosbivur+Daclatasvir in Liver transplant; and "Pharmacologic Considerations when using DAAs in Cirrhosis" at 2013 1st Intl Wkp on the Optimal Use of DAAs in Liver Transplantation April 23, by Jennifer Kiser PharmD
|
| |
| |
[[THIS slide presentation by Jennifer Kiser is below in this report following the ABT450 data;
it was presented in April 2013 just prior to EASL so a little of the info is outdated but note the pk data:
"Pharmacologic Considerations when using DAAs in Cirrhosis"
Jennifer J. Kiser, PharmD, Assistant Professor, University of Colorado Denver
1st International Workshop on the Optimal Use of DAAs in Liver Transplant Patients April 24, 2013]]
Sofosbuvir and Daclatasvir Combination Therapy in a Liver Transplant Recipient With Severe Recurrent Cholestatic Hepatitis C - (11/25/13)
Drug-drug interactions with oral anti-HCV agents and Idiosyncratic Hepatotoxicity in the Liver Transplant setting - (11/25/13)
Single-Dose Pharmacokinetics of Daclatasvir (DCV; BMS-790052) in Subjects With Hepatic Impairment Compared With Healthy Subjects
http://www.natap.org/2011/AASLD/AASLD_78.htm
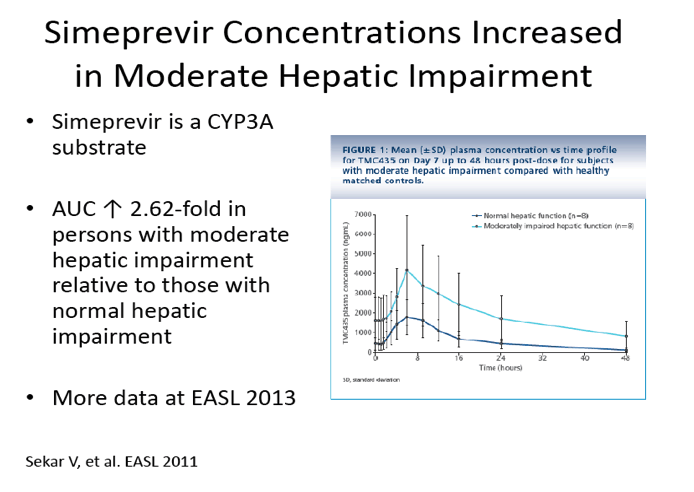
Pharmacokinetics of TMC435 in subjects with moderate hepatic impairment
http://www.natap.org/2011/EASL/EASL_115.htm
Assessment of HIV Antiretroviral Drug Interactions With the HCV NS5A Replication Complex Inhibitor Daclatasvir Demonstrates a PK Profile Which Supports Coadministration With Tenofovir, Efavirenz and Atazanavir/r
http://www.natap.org/2012/CROI/croi_38.htm
The Effect of Hepatic Impairment on the Safety, Pharmacokinetics, and Antiviral Activity of GS-7977 in Hepatitis C Infected Subjects Treated for Seven Days
http://www.natap.org/2012/EASL/EASL_47.htm
Asunaprevir Pharmacokinetics and Safety in Subjects With Impaired Renal Function
http://www.natap.org/2013/AASLD/AASLD_114.htm
Reported by Jules Levin
AASLD 2012
Amit Khatri1, Isabelle A. Gaultier2, Rajeev Menon1, Thomas C. Marbury4, Eric Lawitz5, Thomas J. Podsadecki2, Victoria Mullally3, Walid M. Awni1, Barry Bernstein2, Sandeep Dutta1
1Clinical Pharmacology and Pharmacometrics, Abbott Laboratories, Abbott Park, IL, United States; 2Global Pharmaceutical Research and Development, Abbott Laboratories, Abbott Park, IL, United States ; 3Clinical Program Management, Abbott Laboratories, Abbott Park, IL, United States; 4Orlando Clinical Research Center, Orlando, FL, United States; 5Alamo Medical Research, San Antonio, TX, United States

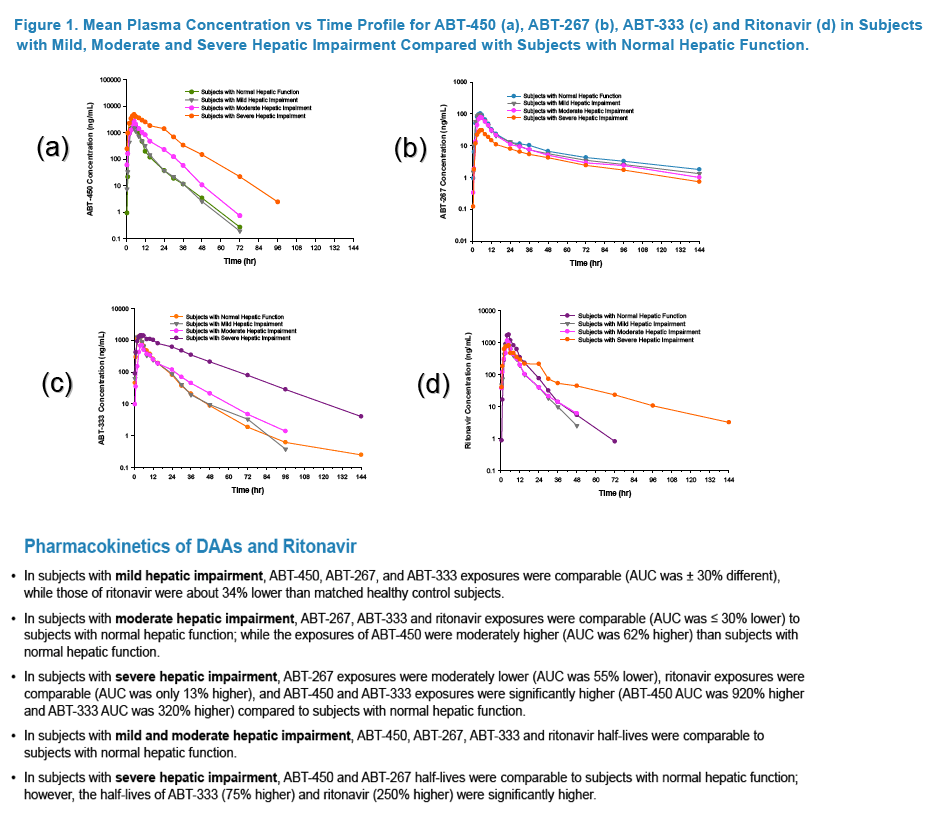




|
| |
|
 |
 |
|
|