 |
 |
 |
| |
Clinical Pharmacology at CROI 2012
PrEP, HCV, Cure(residual replication in reservoirs), TB-dolutegravir, orally inhaled steroids, GS-7340, older age CSF ART levels, TDF levels/bone/renal
|
| |
| |
Courtney V. Fletcher, Pharm.D.
Dean and Professor
College of Pharmacy
University of Nebraska Medical Center
986000 Nebraska Medical Center
Omaha, NE 68198
The 19th Conference on Retroviruses and Opportunistic Infections (CROI) was held in Seattle, WA from March 5 to March 8, 2012. CROI continues to be the premier HIV-focused scientific meeting. I believe the major pharmacologic themes and advances that emerged from CROI 2012 were in pre exposure prophylaxis (PrEP), and therapy of hepatitis C virus (HCV) infection or tuberculosis (TB) in persons co-infected with HIV. In this report I will highlight abstracts focused on pharmacologic issues I think are of broad interest or might benefit from some expert clarification. I will discuss abstracts in three broad categories: compartmental penetration of antiretroviral agents, drug-drug interactions, and findings with implications for HIV therapeutics.
Abbreviations
%CV, percent coefficient of variation
ABC, abacavir
ACTG, adult AIDS clinical trials group
APV, amprenavir
ARV, antiretroviral drug
ART, antiretroviral drug therapy
AUC, area under the concentration-time curve
ATV, atazanavir
BOC, boceprevir
Cmin, minimum drug concentration
CNS, central nervous system
COBI, cobicistat
CSF, cerebrospinal fluid
Ctrough, concentration immediately before the next dose
CYP, cytochrome P450 drug metabolizing enzymes
DTG, dolutegravir
DRV, darunavir
ddI, didanosine
EFV, efavirenz
EVG, elvitegravir
FTC, emtricitabine
ETR, etravirine
fAPV, fosamprenavir
IC50, concentration of drug required to inhibit viral replication in vitro by 50%
IDV, indinavir
IM, intramuscular
IQ, inhibitory quotient
3TC, lamivudine
LPV, lopinavir
MVC, maraviroc
NVP, nevirapine
NRTI, nucleoside reverse transcriptase inhibitor
NNRTI, non-nucleoside reverse transcriptase inhibitor
PACTG, pediatric AIDS clinical trials group
PBMCs, peripheral blood mononuclear cells
PD, pharmacodynamic
PG, pharmacogenetics/pharmacogenomics
PK, pharmacokinetic
PI, inhibitor of HIV protease
PrEP, pre-exposure prophylaxis
r or RTV, ritonavir
RAL, raltegravir
RBT, rifabutin
RBV, ribavirin
RPT, rifapentine
RIF, rifampin
RPV, rilpivirine
SQV, saquinavir
SC, subcutaneous
TDF, tenofovir disoproxil fumarate
TFV, tenofovir
TVR, telaprevir
TDM, therapeutic drug monitoring
TPV, tipranavir
TB, tuberculosis
ZDV, zidovudine
I. Compartmental Penetration of Antiretroviral Agents
Prevention of sexual mucosal transmission of HIV, particularly to prevent transmission to the young women who bear the brunt of infection in the pandemic's epicenter in Africa is urgently needed to potentially save millions of lives and ultimately end of the pandemic. The protection of HIV acquisition with vaginally administered 1% tenofovir (TFV) gel in the CAPRISA 004 study was an important success, although the 39% rate of protection was suboptimal. In CAPRISA 004, efficacy of the TFV gel was highly and directly correlated with drug concentrations measured in cervicovaginal fluids. In contrast to the protection against HIV acquisition found in CAPRISA 004, the 1% TFV gel arm in MTN003 (VOICE) was recently discontinued following an interim review that found the product was not effective in preventing HIV transmission.
At CROI-2012, the results of the FEM-PrEP trial (abstract 32LB), a randomized, double-blind, placebo-controlled trial of daily oral TDF/FTV in 21020 women were presented. This trial had been discontinued for futility in April 2011, with 33 infections in the TDF/FTC group compared with 35 in the placebo. By self-report, 95% of the TDF/FTC recipients reported they always or usually took the study medication. However, adherence was < 26% based on measured concentrations of TDF in plasma at or near the time of infection. These findings lead to the conclusion that TDF/FTC likely did not work because of suboptimal adherence. CAPRISA 004 and FEM-PrEP highlight two important themes in PrEP (though they apply universally): drugs don't work in patients who don't take them (C. Everett Koop) and there is a relationship between the concentration of drug at the site of action and the effect achieved.
Intracellular TFV-diphosphate concentrations are associated with efficacy in the iPrEx study.
Peter Anderson (Abstract 31LB) and the iPrEx team measured the intracellular concentrations of TFV-diphosphate in men assigned to the oral TDF/FTC arm who acquired HIV infection, matched with active-arm HIV negative controls. They evaluated these results in conjunction with TFV-diphosphate concentrations arising from the STRAND study in HIV negative volunteers of 3 different oral TDF dosing regimens of 2, 4 or 7 doses per week. They found a TFV-diphosphate concentration of > 15.6 fmol/million cells was associated with a ≥ 90% reduction in the risk of contracting HIV compared with placebo. An oral TDF dosing regimen of 2 doses/week was shown to produce concentrations associated with a 76% risk reduction whereas a regimen of 4 doses/week was associated with a ≥ 90% risk reduction of HIV acquisition. This study is important because it provides insights into concentrations of the pharmacologically-active moiety of TDF (TFV-diphosphate) associated with efficacy, and oral dosing regimens that appear necessary to achieve those levels. These data should be informative for the design and implementation, including adherence counseling and measurement, of future PrEP studies.
Cells of the female genital tract have variable uptake of TDF and FTC.
The uptake of TDF and FTC into five different types of cells in the female genital tract was described in Abstract 586. Among the five cell types, a 32-fold range in intracellular TFV concentrations and 64-fold range in FTC concentrations were observed. The greatest uptake for both drugs was seen in the CD4+ HeLa cells, with the lowest in the macrophage THP-1 cells. Historically, the THP-1 cells have been used as representative or a surrogate for the female genital tract. These data raise uncertainty about the best in vitro approach to study and express cell uptake of drugs: is one cell line adequate or would an aggregate of exposure across various cell lines provide a better bridge to clinical trials? Importantly, as the authors concluded, they reinforce the necessity to study drug penetration in vivo.
TFV-diphosphate accumulates in rectal cells of HIV-negative volunteers but not cervical cells, while rectal cell concentrations of FTC-triphosphate are appreciably lower compared with PBMCs.
The intracellular concentrations of TFV-diphosphate and FTC-triphosphate were evaluated in PBMCs, rectal and cervical cells in HIV-negative male and female volunteers (Abstract 587). After 20+ days of oral dosing, TFV-diphosphate concentrations were 16-fold higher in rectal cells compared with PBMC concentrations; concentrations in cervical cells were 3-fold higher. In contrast, FTC-triphosphate concentrations in rectal cells were 5-fold lower compared with PBMCs, while concentrations in cervical cells were equivalent with PBMCs. These data provide compelling evidence for drug specific (i.e. not class, NRTI-specific) penetration and accumulation in various compartments of the body. These data make it tempting to speculate the accumulation of TFV-diphosphate in rectal cells contributed to the efficacy observed in prevention of HIV acquisition among MSM in the iPrEx study, and to wonder if the lack of accumulation for TFV and FTC in cervical cells contributed to the lack of efficacy for oral TDF and FTC in FEM-PrEP and to TDF in VOICE?
The long-acting (LA) rilpivirine formulation achieves concentrations in the female genital tract and rectal tissue in males equivalent to plasma concentrations.
The LA formulation of rilpivirine is being developed as a potential agent for PrEP. Abstract 35 reported the concentrations of rilpivirine in the female genital tract and male rectum over 84 days after a single intramuscular dose to HIV-negative volunteers. In females, genital tract concentrations were equivalent to 20% higher than plasma concentrations over 84 days post dose. Vaginal tissue concentrations, however, were lower than plasma concentrations in assessments out to 28 days post dose. In males, rectal tissue concentrations were equivalent to plasma concentrations at 14 days post dose. These data certainly provide a pharmacologic basis for additional studies, but clearly indicate there is much that needs to be learned about the PK and PD of LA rilpivirine.
Low levels of antiretroviral drugs in duodenal tissue may contribute to residual viral replication.
David Asmuth and colleagues quantified ARV concentrations in plasma and in duodenal tissue obtained from duodenoscopy at baseline at 9 months after initiation of therapy in 16 ARV-naïve persons (Abstract 584). All subjects achieved an undetectable plasma viral load. They reported that concentrations of all the ARVs measured in the various regimens these subjects received (TDF/FTC and either RAL, EFV) were undetectable in duodenal tissue or lower than that in plasma. They suggested the low concentrations of the ARVs in duodenal tissue might be associated with the residual viral replication seen in this compartment, despite undetectable plasma HIV-RNA.
The PrEP clinical trial and compartmental pharmacologic data presented at CROI 2012 highlight several gaps in our knowledge of what determines efficacy of a drug regimen for PrEP. Gaps in our pharmacologic knowledge include the concentrations of candidate agents in mucosal tissues required to prevent HIV infection, the dosing regimen required to achieve those protective concentrations, and the forgiveness of that regimen to variation in use (adherence) by the end user. Furthermore, they highlight the need for strategies to quantify, monitor and achieve high levels of adherence in clinical trials and eventual community use. There is an emerging intersection of data on compartmental penetration generated by investigators in the PrEP field and those in the therapeutic field showing low concentrations of ARVs in cells of lymphoid tissue (see session 33 and Science, December 23, 2011) that concentrations of ARVs in plasma may be poorer surrogates for concentrations in compartments than originally thought. PrEP investigators have conclusively shown the importance of adequate compartmental concentrations. The treatment field needs to catch up; I believe overcoming these pharmacologic barriers will be an essential element of strategies for a sterilizing or functional cure.
II. Drug-Drug Interactions
Concomitant administration of boceprevir (BOC) and antiretrovirals: some combinations are OK and some are not recommended.
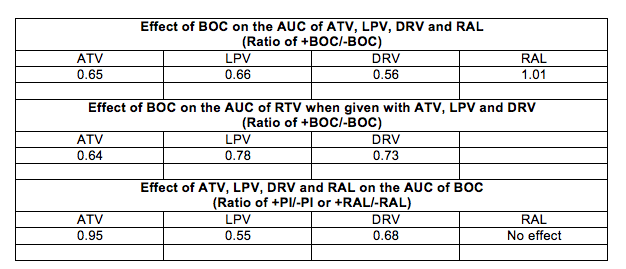
Abstracts 771LB and 772LB reported important drug-drug interactions studies with the HCV protease inhibitor BOC when given with antiretroviral agents (ARVs) in healthy volunteers. Abstract 771LB evaluated BOC with ritonavir-boosted ATV, LPV or DRV, and abstract 772LB the effect of BOC with RAL. I have combined the results of these 3 studies into the table below.
These results show the following:
· BOC reduces the concentrations of all PIs from 34% to 44%, but has no effect on RAL
· BOC reduces the concentration of RTV
· LPV and DRV decrease BOC concentrations by 32% to 45%, while ATV and RAL do not effect BOC concentrations.
Plus,
· An earlier drug interaction study found that EFV reduced the AUC of BOC by 19% and the trough concentration by 44%, while BOC slightly increased, by 20%, the AUC of EFV. The current FDA-approved label recommends that BOC and EFV should not be coadministered.
So, what observations can we draw from these drug interaction data.
· First, that the drug interaction potential with BOC is far greater than initially thought. In my report from CROI 2011, I wrote that I suspected this and these interaction studies bear this out.
· Second, based on the study with BOC and RAL, there is no evidence for an interaction and coadministration should be OK.
· Third, the effect of LPV/RTV and DRV/RTV on BOC concentrations is greater than that of EFV. If coadministration of BOC and EFV is not recommended, then coadministration with LPV/RTV or DRV/RTV should not be recommended either.
· Fourth, the decrease in ATV, LPV and DRV concentrations when given with BOC also make a case for a recommendation to not coadminister. This recommendation was included in a Dear Health Care Provider letter of February 6, 2012 from Merck, Inc.
Collectively then, based on the pharmacokinetic data presently available, I believe the recommendations for coadministration of BOC with antiretroviral regimens would look like this:
Regimen Components Recommendation for coadministration of boceprevir (BOC)
ATV/RTV, LPV/RTV or DRV/RTV Coadministration of BOC is not recommended
EFV Coadministration of BOC is not recommended
RAL BOC coadministration acceptable
Complicating the interpretation of these data, however, are the results of a small phase II clinical trial presented at CROI 2012 (Abstract 47) of BOC or placebo combined with pegylated interferon and ribavirin in HIV infected persons who were receiving ARV therapy with an HIV RNA level in plasma < 50 copies/mL. The overall results were very compelling that the addition of BOC improved the response to HCV therapy. The rate of sustained HIV virologic response 12 weeks after completion of therapy (SVR12) was 60.7% for the BOC recipients compared with 26.5% for those who received placebo. This trial was started prior to the availability of the drug interaction data reviewed above with HIV protease inhibitors. 45 (74%) of the 61 recipients of BOC were receiving an ARV regimen containing ATV/RTV, LPV/RTV or DRV/RTV. In the presentation of this study, the authors reported that rebound of HIV-RNA was seen in 7 participants, 3 receiving BOC (and all 3 receiving ATV/RTV or LPV/RTV) and in 4 who were receiving the BOC placebo.
These results could argue that BOC could be administered safely and efficaciously with a RTV-boosted PI in some patients. But, I would not recommend this approach, at least at this time. First, we need to learn what the PK interactions among BOC and the HIV PIs looks like in co-infected persons who will receive these drugs, and whether there are any differences compared with healthy volunteers. Second, we need to see if any information can be gleaned from the phase II study presented at CROI about characteristics of these patients that might have conferred some mitigating influence on the interaction between BOC and the PI the patient was receiving. Finally, with the PK data available indicating no adverse interaction between BOC and RAL, therapeutic strategies are available to rigorously compare both HCV and HIV treatment responses of a BOC and RAL containing regimen with that of a BOC and PI containing regimen for safety and virologic efficacy.
There is an adverse drug interaction between the investigational HCV protease inhibitor TMC435 and EFV, but coadministration with RPV, TDF and RAL looks OK.
TMC435 is an investigational HCV protease inhibitor. Abstract 49 reported the results of drug-drug interactions studies in healthy volunteers between TMC435 and rilpivirine (RPV), TDF, RAL and EFV. The ratio of TMC435 trough concentration when given with the ARV agents compared with alone were: RPV, 0.96; TDF, 0.93; RAL, 0.86 and EFV, 0.09. These results indicate no adverse interaction likely to be clinically significant with RPV, TDF and RAL. However, EFV substantially reduces TMC435 concentrations and coadministration will not be possible. The effect of TMC435 on the concentrations of these ARVs was also evaluated, and there was no significant interactions were observed.
On the forefront of treating HIV and TB, promising results from two important drug-drug interactions studies with dolutegravir and rifampin, and raltegravir and rifapentine.
Dolutegravir (DTG) in an HIV integrase inhibitor presently in late stage clinical development at a dose of 50 mg once daily (see Abstract 102LB for the 96-week results of a phase 2b study). The results of a drug-drug interaction study between DTG and rifampin (RIF) were reported in Abstract 148. With the dose of DTG increased to 50 mg twice daily, and coadministered with RIF, 600 mg once daily, the concentrations of DTG were similar to those of DTG at 50 mg once daily and no RIF: the ratio (+RIF to -RIF) for AUC was 1.33 and 1.22 for DTG trough. Clinical validation of safety and efficacy is necessary, but these results look promising for a much-needed therapeutic strategy for treatment of HIV and TB coinfection.
The interaction between RAL and rifapentine was described in Abstract 615. In this healthy volunteer study the PK of RAL, 400 mg twice daily, was evaluated with 2 different RPT regimens, 900 mg once weekly and 600 mg once daily for 5 of 7 days per week. When RAL was given with once weekly RPT, the trough concentration of RAL was unchanged, while AUC and Cmax were actually increased by approximately 70%. When RAL was given with daily RPT, AUC and Cmax were unchanged, but the RAL trough concentration was decreased by 40%. The results from this study indicate that RPT has less of an inductive effect than does rifampin (RIF), which substantially decreased RAL concentrations with a 40% decrease in AUC and a 60% decrease in trough. The PK data would suggest that coadministration of RAL at the usual dose and once weekly RPT should be acceptable. I am less sure about coadministration of RAL at the usual dose with daily (5 of 7 days per week) RPT. The 40% on average decrease in RAL trough concentrations and the interpatient variability in RAL concentrations may well place some individuals at risk for subtherapeutic RAL concentrations and virologic failure. Thus, I do not believe the PK data confidently allow a recommendation for coadministration at the usual RAL dose with daily RPT. I think the next step should be a carefully monitored study of the PK, safety and efficacy of RAL and daily dose RPT in co-infected individuals.
Orally inhaled beclomethasone plasma concentrations are not increased by DRV/RTV and the combination does not cause significant adrenal suppression.
With allergy season upon us, and for those who have respiratory disease requiring orally inhaled steroids, Abstracts 610 and 611 presented important PK and PD findings related to the use of an inhaled steroid with a RTV-boosted PI. HIV PIs have been shown to markedly increase plasma concentrations of intranasal fluticasone, and there are several reports of intranasal and orally inhaled steroids resulting in secondary adrenal insufficiency when given with RTV-boosted PIs. These two abstracts are excellent examples of a combined PK and PD analysis of drug-drug interactions. The PK interaction between orally inhaled beclomethasone and RTV (100 mg twice daily) and DRV/RTV (600/100 mg twice daily) was evaluated in healthy volunteers. With just RTV, the AUC ratio (+RTV to -RTV) of beclomethasone plasma concentrations (measured as the active metabolite beclomethasone 17-monoproprionate) was 2.08 and that for Cmax was 1.67. These data indicate that 100 mg twice daily of RTV doubles beclomethasone plasma concentrations. However, these ratios when DRV/RTV was given were 0.89 for AUC and 0.81, indicating a slight reduction in beclomethasone plasma concentrations. These results indicate that DRV/RTV does not increase beclomethasone plasma concentrations. Furthermore, they provide a very nice example of a difference in direction and magnitude of the interaction between DRV/RTV and RTV.
Abstract 610 provided the results of baseline cortisol levels and the adrenocorticotropic hormone (ACTH) stimulation test as assessments for adrenal suppression. No significant difference was seen in basal and maximum cortisol levels with either 28 days of RTV or DRV/RTV, indicating there was no adrenal suppression.
Collectively, these data indicate orally inhaled beclomethasone may be safely given with DRV/RTV (600/100 mg twice daily). I believe these findings should extrapolate to intranasal beclomethasone as well.
III. HIV Therapeutics
Older age was associated with higher ARV concentrations in CSF.
Numerous issues are emerging with regard to HIV and aging. Abstract 592 reported results from 209 HIV-infected persons where TFV, EFV and ATV concentrations in the CSF increased with increasing age. There appeared to be some evidence for higher ARV concentrations in the CSF and an association with worse neurocognitive functioning. These findings are provocative and certainly warrant additional studies in older persons.
Plasma HIV RNA decreased to a greater degree with the tenofovir prodrug, GS7340, compared with standard dose TDF.
GS7340 is a prodrug of TFV that achieves higher intracellular concentrations from lower plasma concentrations, and therefore is more potent than TDF. For example, the IC50 for TFV is 1.2 μM, 0.015 μM for TDF, and is 0.003 μM for GS-7340. Abstract 103 described a 10 day monotherapy study in 38 HIV-infected persons who received placebo, TDF (300 mg once daily) or GS7340 (8mg, 25mg or 40 mg). The median changes in HIV-RNA from baseline to day 10 (log10 copies/mL) were: placebo, 0.003; TDF, -0.97; GS7340 8mg, -1.08; GS7340 25mg, -1.59; and GS7340 40mg, -1.73. Plasma concentrations of TFV were reported to be 80+% lower with GS7340 than those from administration of TDF. GS7340 represents a very interesting strategy: a prodrug that achieves lower plasma concentrations, so hopefully fewer adverse effects, but higher intracellular concentrations and hopefully improved long-term suppression of HIV. Because GS-7340 requires a lower dose of TFV to achieve a greater suppression of HIV, the cost of goods should be lower, so hopefully it will be less expensive? This is an intriguing drug - more to come.
And sticking with the tenofovir theme are three abstracts describing TDF-associated decreases in bone mineral density (BMD) and kidney toxicity.
Abstract 125LB investigated changes in bone metabolism in persons with virologic suppression on an AZT/3TC-containing regimen who switched to TDF/FTC or remained on AZT/3TC. 48 weeks later, all measures of bone formation and resorption were significantly increased in the TDF/FTC recipients. Additionally, there were significantly greater decreases in BMD measures in the lumbar spine. Collectively, these data indicate a change from AZT/3TC to TDF/FTC was associated with increases in bone turnover that correlate with decreases in BMD. This is a complication of TDF therapy I think warrants additional investigation and monitoring.
Abstract 603 investigated the association between kidney tubular dysfunction (KTD) and plasma concentration of TFV. In 161 persons, 17 (11%) met the definition of KTD; they had higher 12-hour TFV plasma concentrations than those subjects who did not: 143 ng/mL vs. 108 ng/mL. Additionally, those with KTD had a longer duration of TDF exposure. These data indicate higher plasma concentrations of TFV were associated with an increased risk for developing KTD, although a clear threshold concentration could not be identified. The most important take home messages for clinicians from this work, is the need to make sure the TDF dose is adjusted in subjects who have renal insufficiency (or use an alternative agent) and to monitor renal function carefully in patients who are receiving concomitant drugs known to increase TFV plasma concentrations.
Additional insight into patient characteristics that increase TFV plasma concentrations was provided in Abstract 604. In 222 patients on TDF-containing ARV therapy, the following characteristics were related with increased TFV plasma concentrations: increasing age, decreasing creatinine clearance, and decreasing body mass index. Additionally, concomitant therapy with an unboosted PI (primarily ATV) was associated with elevated TFV plasma concentrations. These data suggest the older, small body weight person with some degree of renal insufficiency should receive very close monitoring for renal safety and tolerability if they are receiving TDF-containing ART.
Closing Comments - a time for optimism.
At CROI 2012, work was presented that described important advances in development and use of existing and new, drugs and regimens for prevention and for treatment of HIV in children, adolescents and adults. Most illustrative as to the current state of HIV pharmacotherapy are the results presented in Abstract 137 from the NA-ACCORD study that examined life expectancy from 1996 to 2007 in 65,484 HIV-infected persons. I quote: "a 20-year old individual with HIV today in North America would expect to live into their early 70s, a life expectancy only slightly lower than that of a person in the general US population". These results coupled with scientifically grounded presentations on strategies, and yes some challenges, for a sterilizing or functional cure for HIV infection add to the sense of optimism. Truly, I believe a time for optimism, but, no time for complacency - - there remains much to do.

|
| |
|
 |
 |
|
|