 |
 |
 |
| |
Lack of Resistance Development to the HIV-1 Attachment Inhibitor BMS-626529 During Short-Term Monotherapy with its Prodrug BMS-663068
|
| |
| |
Reported by Jules Levin
19th Conference on Retroviruses and Opportunistic Infections. March 5-8, 2012. Seattle, WA.
Neelanjana Ray1, Megan Wind-Rotolo1, Matthew Healy2, Carey Hwang1, Shu-Pang Huang1, Jeannette Whitcomb3, Nannan Zhou2, Mark Krystal2, George J. Hanna1
1Bristol-Myers Squibb Research & Development, Princeton, NJ; 2Bristol-Myers Squibb Research & Development, Wallingford, CT; 3Monogram Biosciences Inc., South San Francisco, CA
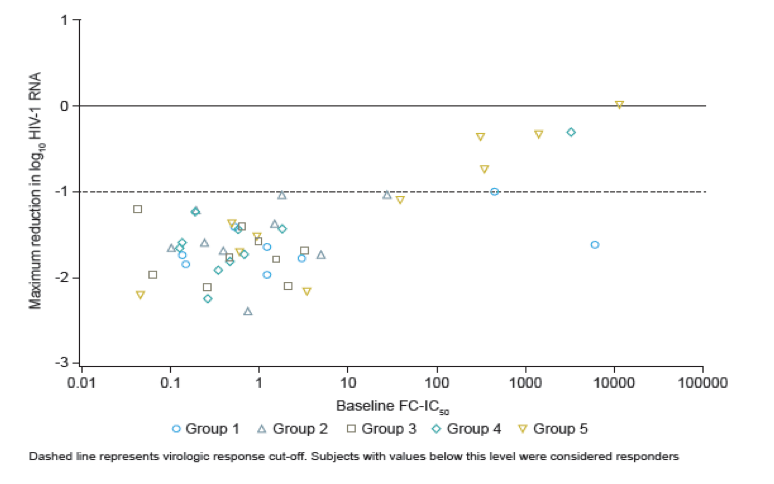
Low baseline susceptibility to BMS-626529 (reflected by a high baseline FC-IC50) was predictive of virologic non-response to its prodrug BMS-663068.
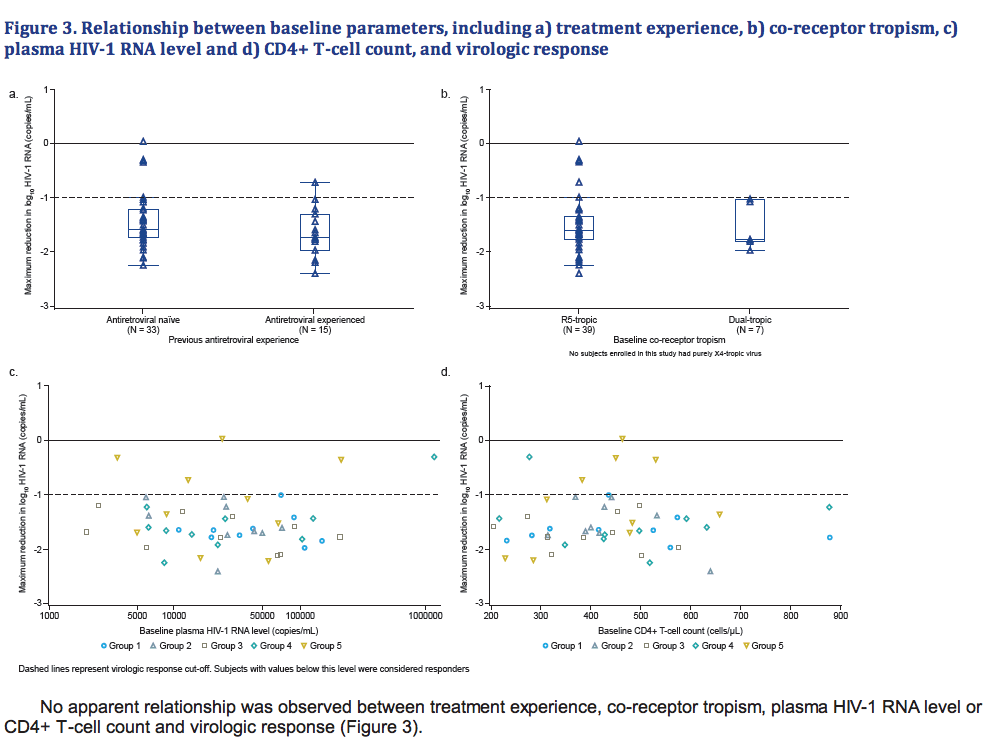
- There was no apparent relationship between prior treatment experience, baseline co-receptor tropism, baseline plasma HIV-1 RNA level or baseline CD4+ T-cell count, and virologic response.
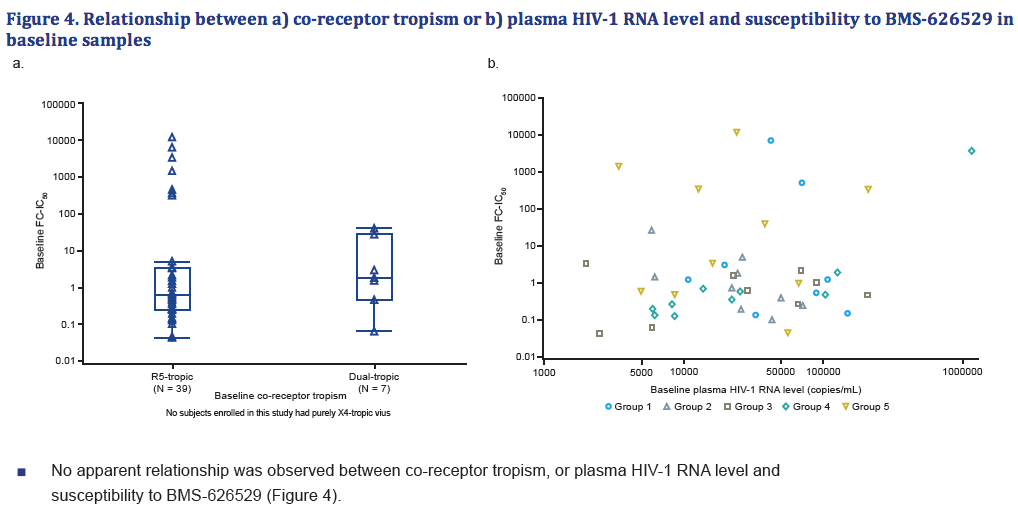
- There was no apparent relationship between co-receptor tropism or plasma HIV-1 RNA level and susceptibility to BMS-626529.
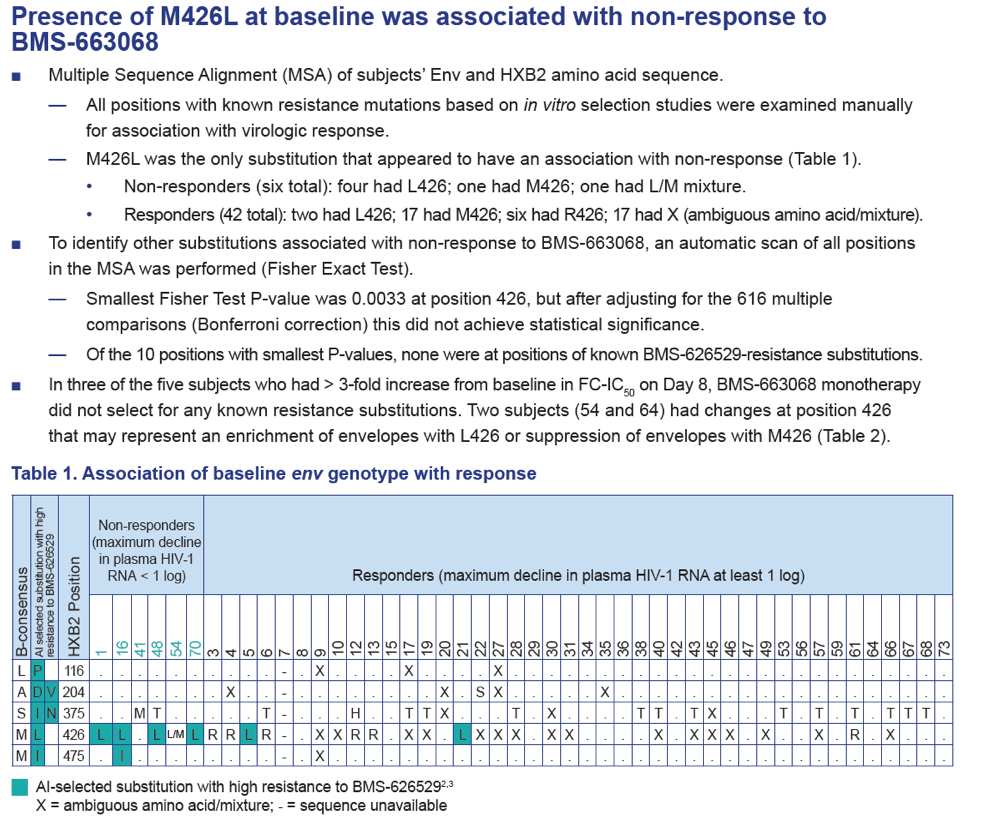
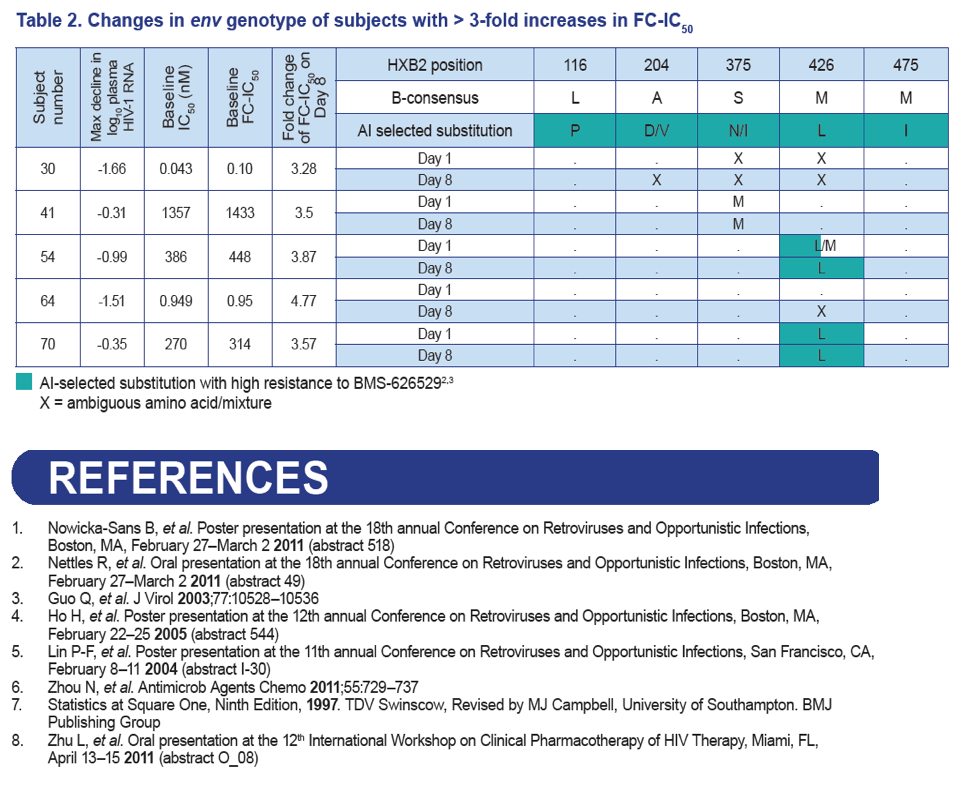
- Presence of env M426L substitution at baseline was associated with non-response to BMS-663068.
Monotherapy with BMS-663068 for up to 8 days did not appear to select for BMS-626529 resistance.
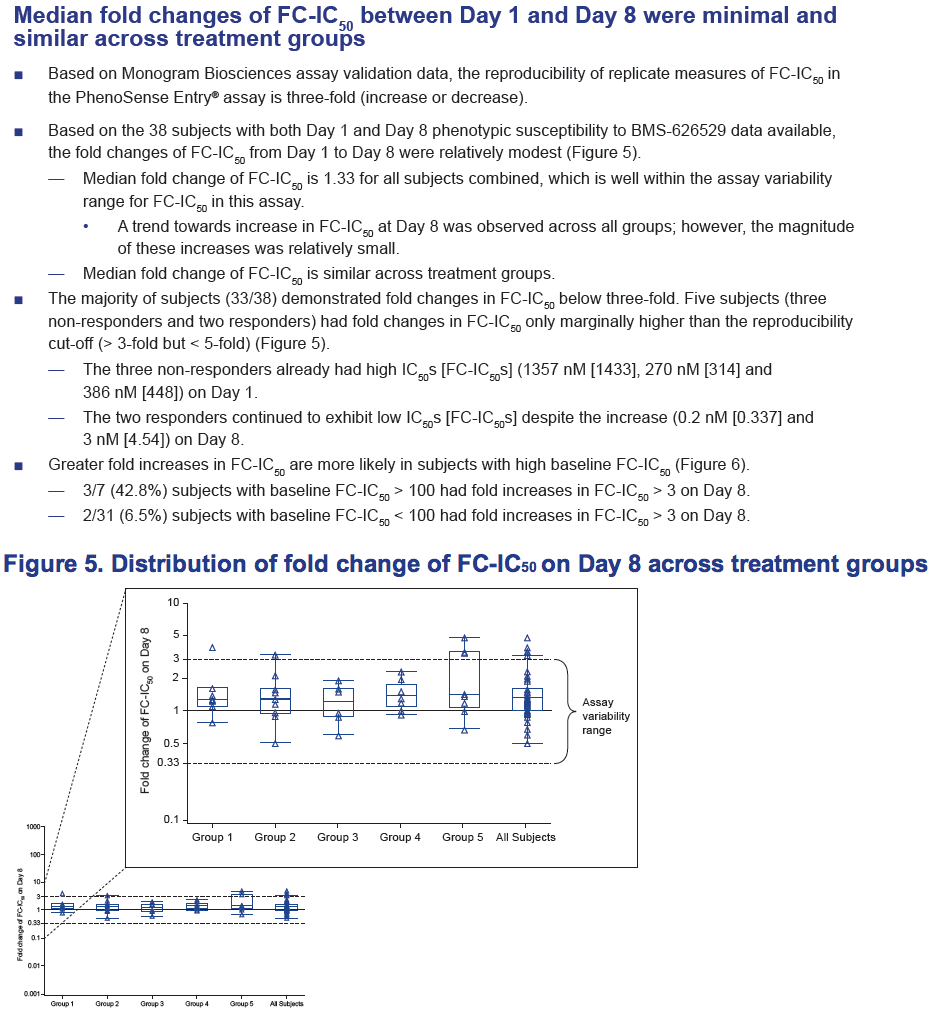
- Median fold change of FC-IC50 was minimal, and similar across treatment groups.
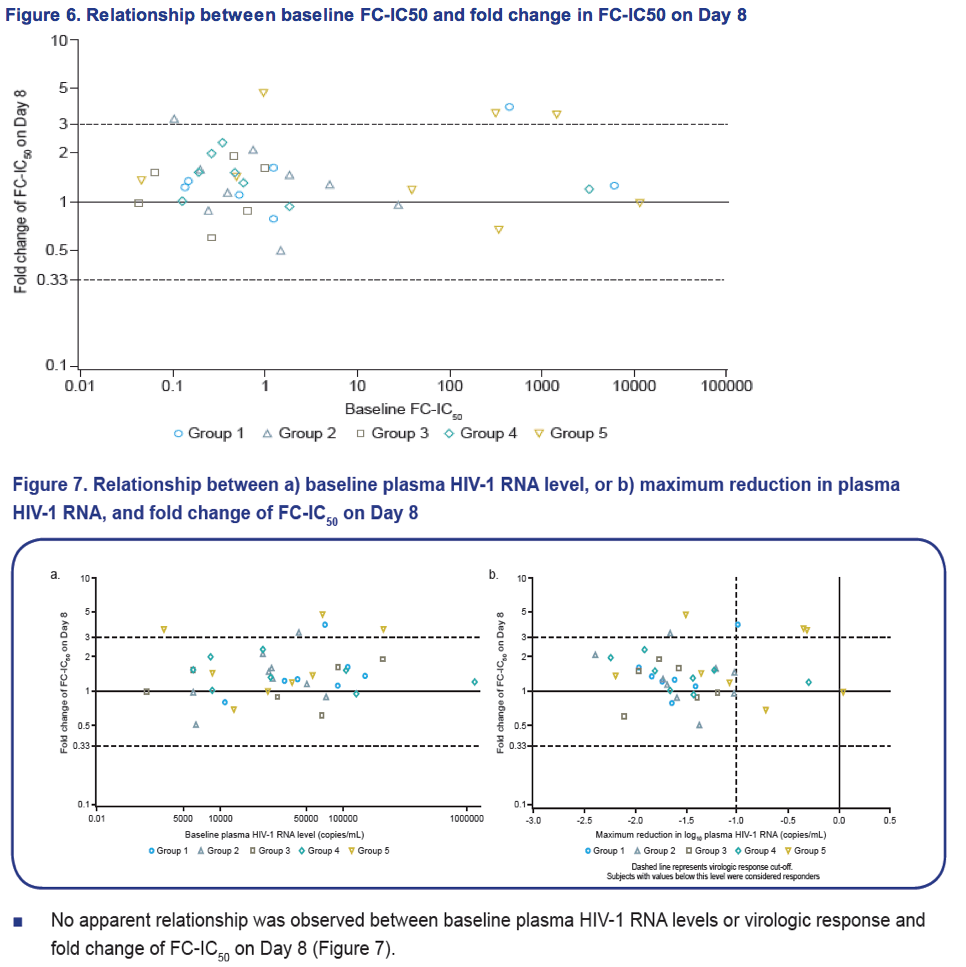
- There was no apparent relationship between baseline plasma HIV-1 RNA levels or virologic response and fold change of FC-IC50 on Day 8.
- Possible increases in the proportion of L426 were observed in the envelope populations for two subjects and may represent the effect of drug pressure by BMS-663068.
- However, no clear selection of a known AI resistance mutation was observed through population sequencing during the 8-day course of BMS-663068 monotherapy.
ABSTRACT
Background: BMS-626529 is a first-in-class oral HIV-1 attachment inhibitor (AI) that binds to gp120 and impedes the first step of viral entry by preventing gp120 engagement of the CD4 receptor. In a randomized, open-label, multiple-dose, 8-day monotherapy study, BMS-663068, the prodrug of BMS-626529, demonstrated potent antiviral activity. The present analysis examines changes in phenotypic susceptibility and known AI- resistance substitutions during this short-term monotherapy study.
Methods: Phenotypic susceptibility of subjects' viral quasispecies at Days 1 (D1) and 8 (D8) were assessed from plasma using the PhenoSense Entry® assay (Monogram). To minimize inter-assay variability, subjects' IC50s were normalized to that of a control virus to obtain fold-change IC50 (FC-IC50). Population gp160 sequencing of D1 and D8 samples was performed using GeneSeq® Env (Monogram).
Results: Of the 48 subjects who completed the study, there were 42 responders (> 1.0 log10 copies/mL decline in HIV-1 RNA) and six non-responders. All non-responders had baseline IC50 > 100 nM. Phenotypic susceptibility to BMS-626529 could be obtained for 46 subjects on D1 and 38 subjects on D8. Based on the 38 subjects with both D1 and D8 data available, the fold changes of FC-IC50 from D1 to D8 were relatively low (median 1.33, range 0.51-4.77).In 33/38 subjects (86.8%) the fold changes of FC-IC50 from D1 to D8 were less than three-fold, which is the fold change reproducibility cut-off for the PhenoSense Entry® assay. In 5/38 (13.2%) subjects (three non-responders and two responders), D8 fold changes in FC-IC50 were marginally higher than the reproducibility cut-off (> 3-fold but < 5-fold). However, the three non-responders already had high IC50s at baseline (1357 nM, 270 nM and 386 nM on D1) and the two responders exhibited low IC50s despite the increase (0.2 nM and 3 nM on D8). Population gp160 sequencing at baseline revealed an association of the known M426L AI resistance substitution with high IC50 and poor virologic response (5/6 non-responders and 2/42 responders harbored 426L). Other minor AI resistance substitutions were also present at baseline in certain non-responders with or without M426L. In the 39 subjects with both D1 and D8 gp160 population sequencing data available, none demonstrated clear selection of a known AI resistance mutation at D8.
Conclusions: Monotherapy with BMS-663068 for up to 8 days did not appear to select for BMS-626529 resistance.
NOTE: This abstract has been updated with final fully validated data.

|
| |
|
 |
 |
|
|