 |
 |
 |
| |
NeuroAIDS at CROI 2012
|
| |
| |
David Alain Wohl, MD - The University of North Carolina
Introduction
HIV can be bad for the brain. Before advanced HIV therapy became available, those with AIDS often suffered doubly from deterioration that was physical and cognitive. But now, with longevity that approaches that of the HIV-free, people who are living with the virus rarely experience dementia or severe impairments in thinking. This is not to say that potent ART has completely eradicated the neurological effects of HIV. In fact, a number of studies have found a high prevalence of neurocognitive impairment among HIV-infected patients when applying sophisticated neuropsychiatric testing batteries. Fortunately, these data indicate that although some sort of neurological impairment is detected in a third to half of HIV+ people, the overwhelming majority of these individuals have mild involvement or may even be completely asymptomatic.
Thus, given the lion's share of cognitive impairment is found to be very mild on sensitive tests, the challenge for the NeuroAIDS field is to determine how significant this problem really is, and the natural history and the determinants of decline in neurocognition in those with HIV. Addressing these issues is required before interventions to prevent and treat cognitive impairment associated with HIV can be developed. At this year's conference there were a several sessions on NeuroAIDS that grappled with these fundamental questions. Highlights are described below.
Predicting Cognitive Impairment
NeuroAIDS-ologists are now all reading from the same playbook. At a meeting held in Frascati, Italy in 2007, the experts in the field agreed to a new classification system of neurological impairment, ranging from none to dementia (1). This common lexicon, christened with a name straight out of a Dan Brown novel, the Frascati Criteria, has helped to standardize how neurocognitive impairment in the setting of HIV is described.
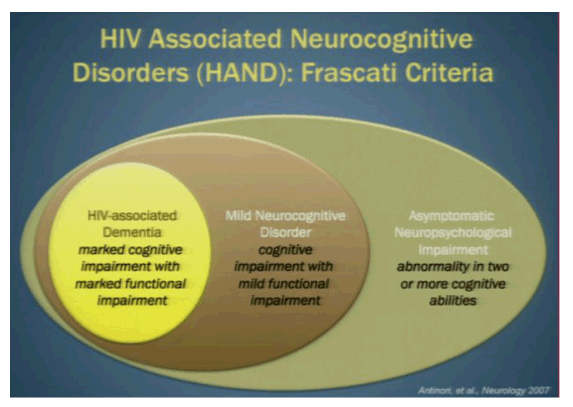
In the classification system, a notch below normal is "asymptomatic neurological impairment (ANI)" in which the individual has abnormalities in two or more cognitive abilities. As the majority of impairment is asymptomatic, there is considerable interest in determining whether those on this end of the Frascati spectrum are at risk of progressing to more severe disease over time. Investigators from the CHARTER cohort, a large collection of HIV+ patients followed longitudinally and subjected to neurocognitive testing at regular intervals, examined normal and ANI classified participants to assess changes in self-reported and objectively measured functional decline (2).
A total of 347 HIV+ participants with a median of 45 months follow-up were included. Of these, 226 normal tested as normal on the neurocognitive tests (median CD4 459, nadir CD4 201) and 121 as ANI (CD4 425, nadir CD4 162). Two thirds of those with normal cognition and 72% of those with ANI were on ART, but the proportion with suppressed HIV viral load was not reported or included in the analyses - a major problem with these results, as discussed below.
A decline in function was seen in both groups over time but was more profound for those with ANI. The relative risk for decline to functional impairment for those with ANI compared to those testing normal was 2x higher on self-report, over 5x higher for performance decline and 3x when the combined self-reported or performance testing was used. Baseline predictors of decline to functional impairment included: older age, being a woman, substance abuse, comorbid conditions such as HCV, AIDS, and low nadir CD4. Time dependent covariates that were associated with functional decline were CD4 and depression. Interestingly, the Central Nervous System Penetration Effectiveness (CPE) score that is intended to rank antiretroviral drug levels in the brain was not associated with decline in functioning.
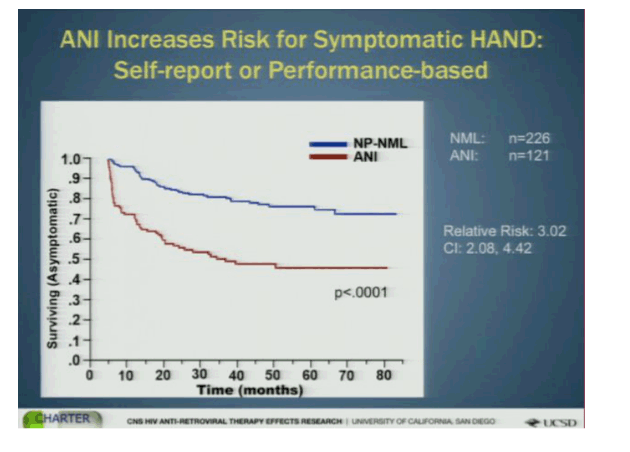
Bottom Line: Those with ANI were at greater risk of developing impairment in functioning. However, the exact types of functional problems were not described and so it remains unclear how meaningful these declines were clinically. Importantly, viral suppression not assessed and while most of these participants were being treated for HIV it is known that uncontrolled viremia is associated with neurocognitive problems. Imbalances in the proportion with controlled HIV could confound the results. Further these and other NeuroAIDS researchers have been unable to guide clinicians in how best to incorporate their warnings about cognitive decline into everyday practice. For instance, these results, suggesting ANI is a useful designation that carries a significant risk of development of functional decline, are important but need to be further analyzed to help make it clearer what the real life risks are for patients living with HIV. Dr. Igor Grant, who presented the study, ended by recommending that patients with ANI should be monitored closely for declines in function. But, how should we know who has ANI? As sophisticated neuropsychological testing requires trained staff to administer and interpret it is not part of routine practice. Further, how exactly should patients be monitored and to what ends exactly? Recommendations that include techniques for assessment that can be clinically applied would be most helpful. Clearly, more needs to be done by this field to help clinicians and patients put these findings to good use.
Early HIV and Effects on Brain
Much of the data regarding HIV-associated cognitive neurocognitive disorders has been collected from patients with established HIV infection. Much less is known about how early HIV infection impacts thinking. Julia Peterson from UCSF presented data from an observational study of 70 patients who had recent acute HIV infection and underwent extensive neuropsychological testing at baseline (about 4 months after estimated HIV infection), six weeks later and then every six months, thereafter (3). Importantly, some patients chose to initiate ART during, while others did not.
As expected, all were men, and the median CD4 cell count was high at 555/mm3. The majority had a history of substance abuse in the past and most had used drugs in the month prior to their study testing.
At baseline, 42% were categorized as having ANI (asymptomatic neurocognitive impairment) or MND (mild neurocognitive impairment). When comparing the 33 subjects who started ART (at a median of 31 weeks after infection) with those who did not initiate therapy, testing revealed that there was declines in scores for motor function, but not other domains, in those not treated which was progressive. However, among those on ART, motor performance scores stabilized and did not progress once treatment was started. Learning and processing speed actually improved in both groups over time.
Bottom Line: This study of those with early HIV infection found a high prevalence of asymptomatic/mild impairment. However, there were no data available from prior to or just after infection to determine if this was present before HIV acquisition. This was a group of hard living men with significant substance abuse disorders and possibly mental illness. The performance scores were graded in comparison to a "normal" control population that may not be well suited to such men or other men at risk for HIV infection today. ART had some effect of stabilizing motor declines as measured by tests like the groove peg board, finger tapping and timed gait, but not anything else. As these patients were not randomly assigned to start HIV therapy therapy there may have been some bias in that those selected to start treatment may have been less likely to experience further impairments (i.e., those with less substance abuse or depression). More work is needed to understand whether early HIV infection changes thinking and functional performance.
Other Studies:
Several poster presentations provided bits of data that may be useful when considering HIV-related neurocognitive issues. For example, a study presented by Allen McCutchan from UCSD looked at associations between body shape and other metabolic parameters and neurocognitive among 130 participants in the CHARTER cohort (4). The major finding was that those with cognitive impairment had a larger average waist size (by approximately 4 inches) compared to those deemed normal on neurocognitive testing. In a confusing multivariable model that included waist circumference and body mass index (BMI), despite the correlation of the two, higher BMI was found to be protective against cognitive impairment (even though wasit size was detrimental). Diabetes was associated with increased risk of impairment. These results require more thought and possible greater analyses. Waist circumference may or may not be a marker of central obesity, as the investigators state, but an indication of generalized obesity. It will be interesting to see how these data are presented once peer-reviewed.
In another study, that can be filed under 'tell us something we don't already know'; a group from Johns Hopkins found that aging makes HAND worse (5). Those patients who were over 50 years of age had worse memory problems than those younger. To be fair, documenting what seems to be common sense has value and undoubtedly age will be a major factor in the cognitive function of our patients.
Summary
The field of NeuroAIDS seems to be still getting its footing after potent antiretrovirals came along and made scarce the kind of AIDS dementias seen in the pre-HAART era. The good news is that apparently many of our patients with neurocognitive disorders the signs of something amiss are subtle. The bad news is we still do not know how to look for, treat and prevent HAND. We still have few studies of neurocognition in the setting of HIV that can be used when considering the most vexing questions of our time? Does early HIV therapy protect the brain? Will those on effective HIV therapy be protected from HIV-related cognitive impairments? Can we identify the unfortunate few who, due to genetic or other factors, are at heightened risk for neurological decline? How much does it really matter whether antiretrovirals penetrate the central nervous system? This is what we need to know and while the questions were posed at this CROI, there were few firm answers.
References
1. Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789-99.
2. Heaton R, et al. Asymptomatic Mild HIV-associated Neurocognitive Disorder Increases Risk for Future Symptomatic Decline: A CHARTER Longitudinal Study. Abstract 77. CROI 2012
3. Peterson J, et al. Changes in Neurocognitive Performance from Early HIV-1 Infection to Initiation of ART. Abstract 80. CROI 2012
4. McCutchan A, et al. Role of Central Obesity, Diabetes, and Metabolic Variables in HIV-associated Neurocognitive Disorder. Abstract 490. CROI 2012
5. Tan IL, et al. Older Subjects with HIV Infection Have Increased Memory Impairment Compared to Younger
|
| |
|
 |
 |
|
|