 |
 |
 |
| |
Cardiovascular Risk Tied to MRS-Defined Brain Injury in 45-and-Older Men With HIV
|
| |
| |
19th Conference on Retroviruses and Opportunistic Infections, March 5-8, 2012, Seattle
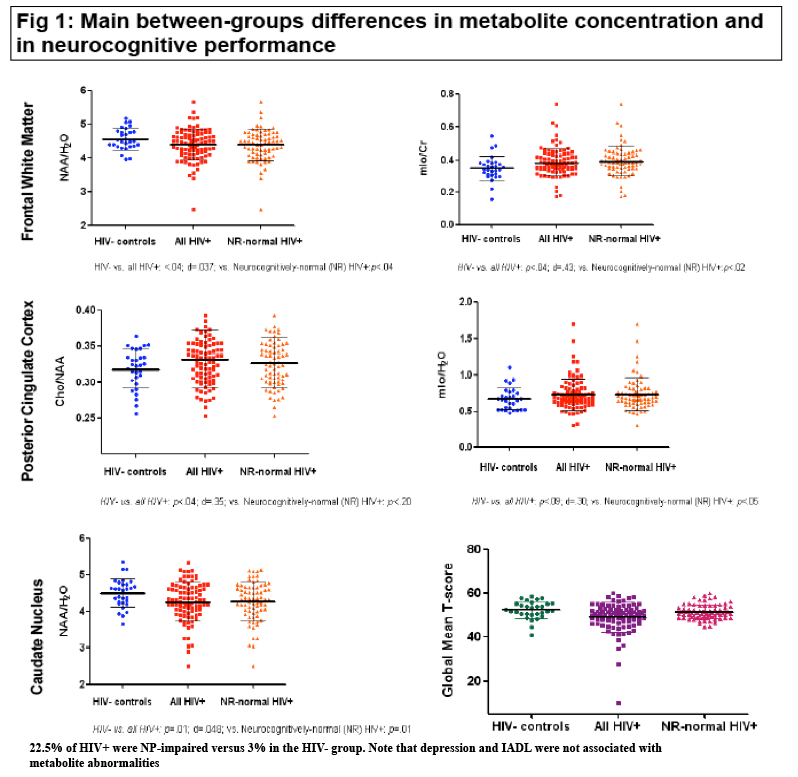
"Rates of overall neuropsychological impairment were 22.5% in the HIV group versus 3% in controls (P < 0.01)."
"There was evidence of accelerated brain aging pathology in the FWM and evidence of CVD-related brain injury in regions that are
typically involved in HIV-related brain injury (FWM and CN) and a region that has been associated with pathological aging (PCC).The
relationship between CSF neopterin with increased FWM and PCC mIo and Cr suggests an active ongoing process of low grade inflammation.
Both the CPE and immune recovery are important for improved brain metabolism. Longitudinal studies will be needed to assess the long-term
effects of CVD and further aging. The enrolment of a HIV-CVD group with CVD will be important to determine whether HIV-CVD truly lead to
greater brain injury over-time."
Mark Mascolini
Framingham-defined 10-year risk of cardiovascular disease risk, duration of HIV infection, and age raised the risk of brain injury defined by magnetic resonance spectroscopy (MRS) to a modest by significant extent in an Australian case-control study involving 92 HIV-positive men with a average age of 56 [1].
Lucette Cysique (University of New South Wales) and colleagues at other Sydney centers planned this study to address three questions: (1) Are HIV infection and age 45 or older associated with increased risk of brain metabolic abnormalities? (2) If HIV-positive people have any brain metabolic abnormalities, do they involve traditionally affected regions of the brain or also regions usually affected in pathological aging (such as the posterior cingulate cortex)? (3) Are brain metabolic abnormalities associated with cardiovascular risks?
The study involved 92 HIV-positive men 45 and older with a nadir CD4 count below 350 and taking a stable antiretroviral regimen for a median of 24 months. The researchers matched them by age and gender to 30 HIV-negative controls. No one in either group had a non-HIV-related neurologic disorder, brain trauma, a psychiatric disorder that involved a psychotic episode, or a substance use disorder. People with depression were not excluded as long as they were clinically stable.
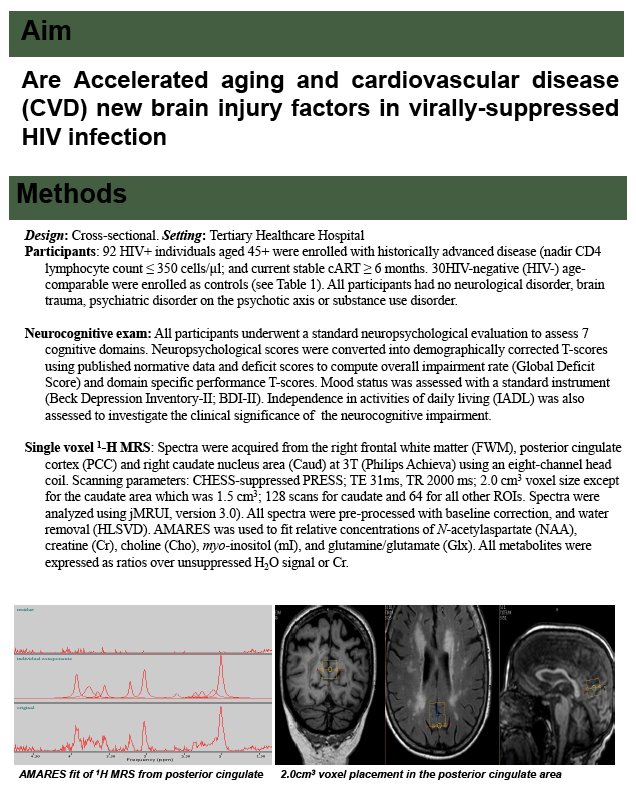
All study participants underwent proton magnetic resonance spectroscopy (1H-MRS) of the right frontal white matter, posterior cingulate cortex, and right caudate nucleus area. The researchers used JMRUI with the AMARES algorithm (a standard tool to quantitate MRS data) to fit relative concentrations of N-acetylaspartate (NAA), creatine, choline, myo-inositol to water, and creatine. They determined cardiovascular risk using the 10-year risk Framingham score (based on the 2008 formula). The study poster, linked at reference 1 below, details the many-layered statistical analyses.
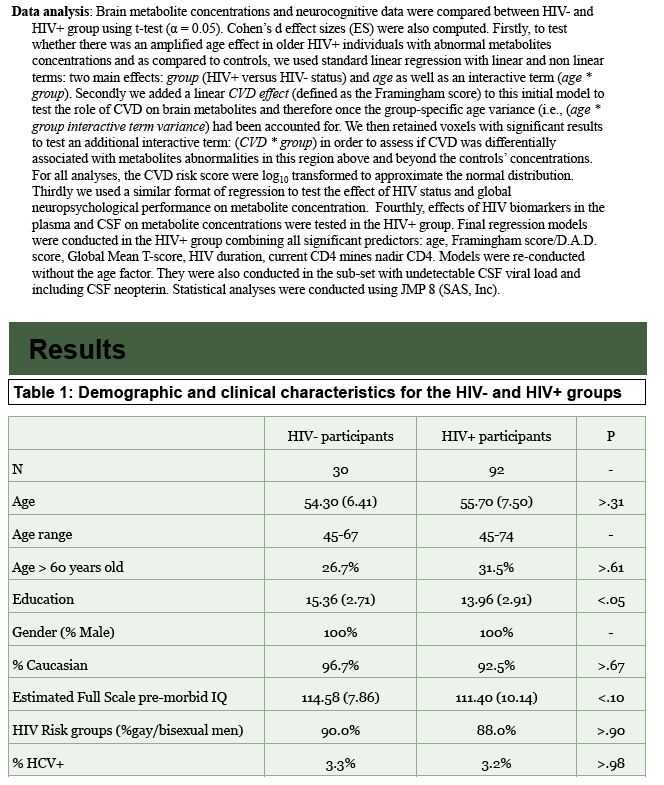
All study participants were men, and more than 90% were Caucasian. Age averaged 55.7 (range 45 to 74) in the HIV group and 54.3 in HIV-negative controls (range 45 to 67). Education was slightly but significantly longer in controls (15.4 versus 14.0 years, P < 0.05), while average estimated full-scale premorbid IQ was marginally higher among controls (114.6 versus 111.4, P < 0.10).
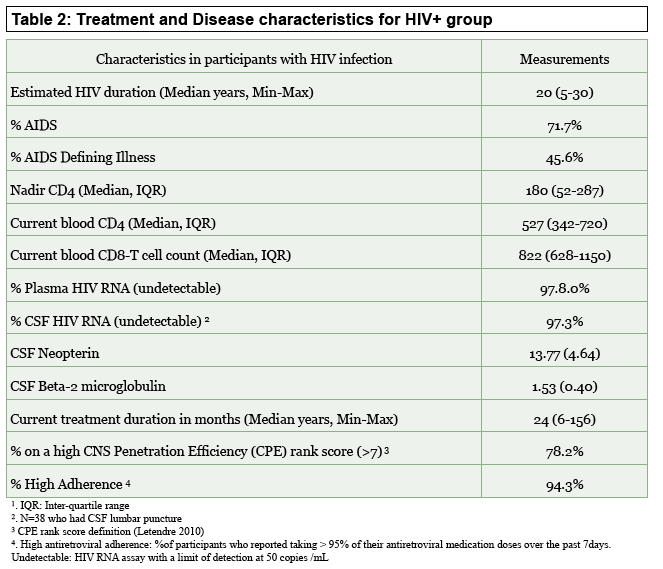
In the HIV group, median estimated infection duration was 20 years (range 5 to 30), median nadir and current CD4 count were 180 and 527, 72% had AIDS, and 98% had an undetectable viral load in blood. Among 38 people with lumbar puncture, 97% had undetectable viral load in cerebrospinal fluid (CSF). Most of these men, 94%, reported greater than 95% antiretroviral adherence.
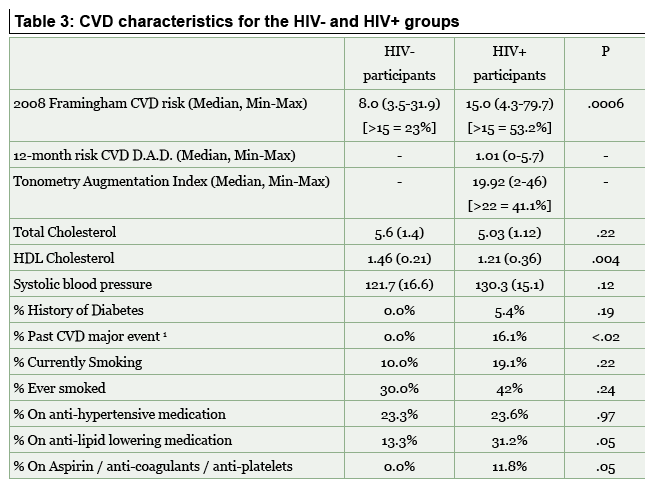
Median Framingham risk score was 15 in the HIV group and 8 in HIV-negative controls (P = 0.0006). Compared with controls, the HIV group had significantly lower "good" high-density lipoprotein cholesterol and marginally higher systolic blood pressure. Proportions of HIV-positive cases and HIV-negative controls were 5.4% versus 0 with a history of diabetes (P = 0.19), 16.1% versus 0 with a major cardiovascular event (P < 0.02), 19.1% versus 10% who currently smoked (P = 0.22), 42% versus 30% who ever smoked (P = 0.22), 31.2% versus 13.3% on lipid-lowering therapy (P = 0.05), and 11.8% versus 0 on aspirin, anticoagulants, or antiplatelets (P = 0.05).
Rates of overall neuropsychological impairment were 22.5% in the HIV group versus 3% in controls (P < 0.01). In frontal white matter, HIV-negative controls had significantly higher NAA/H2O than all HIV-positive people and neurocognitively normal HIV-positives (P < 0.04 for both comparisons) and significantly lower myo-inositol/creatine than all HIV-positives (P < 0.04) and neurocognitively normal HIV-positives (P < 0.02). In the posterior cingulate cortex, HIV-negative controls had significantly lower choline/NAA than all HIV-positive people (P < 0.04) and lower myo-inositol/H2O than all HIV-positives (P < 0.09) and neurocognitively normal HIV-positives (P < 0.05). In the caudate nucleus, HIV-negative controls had higher NAA/H2O than all HIV-positives and neurocognitively normal HIV-positives (P = 0.01 for both comparisons). Further inspection of the data (not detailed in the poster) showed that the slight difference in between-group education and depressive complaints were not associated with those metabolic abnormalities.
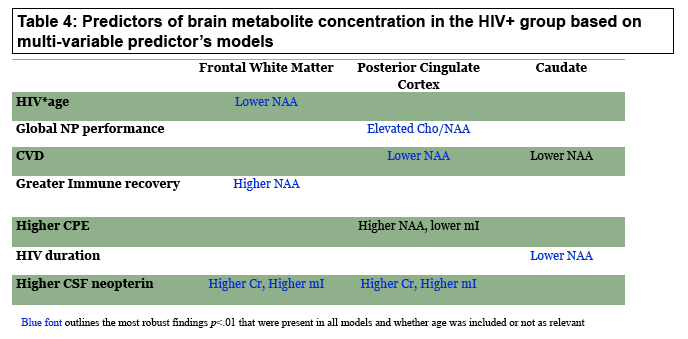
Linear multivariable regression analyses determined that the following variables were significant predictors (P < 0.01) of brain metabolite concentrations in HIV-positive people:
Age-HIV status interaction: lower NAA/H2O in the frontal white matter (and relative to controls)
-- Global neuropsychological performance: elevated choline/NAA in posterior cingulate cortex (and relative to controls)
-- Cardiovascular disease: lower NAA/H2O in posterior cingulate cortex more so when immune markers were included in the regression model and when the chronological age factor was removed
-- Greater immune recovery (current CD4 minus nadir CD4): Higher NAA/H2O in frontal white matter
-- Longer HIV duration: Lower NAA/H2O in caudate nucleus
-- Higher CSF neopterin level: Higher creatine and higher myo-inositol in both the frontal white matter and the posterior cingulate cortex
The researchers concluded that their findings provide "preliminary evidence of accelerated brain aging pathology in the frontal white matter and evidence of cardiovascular disease-related brain injury in regions that are typically involved in HIV-related brain injury (frontal white matter and cingulate cortex) and a region that been associated with pathological aging (posterior cingulate cortex)."
They proposed that the association between CSF neopterin and increased myo-inositol and creatine in frontal white matter and the posterior cingulate cortex "suggests an active ongoing process of low-grade inflammation" in these people with long-term HIV infection and optimal response to antiretroviral therapy.
Cysique and coworkers noted the need for longitudinal studies to assess the long-term effects of cardiovascular disease and further aging on brain injury. Because they found that HIV-positive participants with cognitive deficits have these abnormalities, only a longitudinal study will determine the long-term clinical relevance of these findings. These cross-sectional results can be considered indicative, they proposed, because the magnitude of the brain metabolite abnormalities found were modest, though still significant. The results are consistent with the facts that the study group had low confounding, that most had subclinical cardiovascular disease, and that the rate of neurocognitive impairment was on the lower end of what has been observed in cohorts composed of mostly highly educated men with chronic HIV infection.
In future longitudinal studies, the researchers added, an HIV-negative group with cardiovascular disease should also be included to determine whether the combination of cardiovascular disease and chronic HIV truly lead to greater brain injury over time.
Reference
1. Cysique LA, Moore DM, Moffat K, Carr A, Brew BJ, Rae C. new evidence for cardiovascular-related brain injury in middle-aged HIV+ individuals: A 1-H MRS Study. 19th Conference on Retroviruses and Opportunistic Infections. March 5-8, 2012. Seattle. Abstract 492. http://www.retroconference.org/2012b/PDFs/492.pdf.
New Evidence for Cardio-vascular-Related Brain Injury in Middle-Aged HIV+ Individuals: a 1-H MRS Study
Cysique L A 1 2 3 4;; Moore DM 3 4, Moffat K 3, Carr A3, Brew B J 1 3 4, & Rae C 1 2
1. University of New South Wales, Brain Sciences, Sydney Australia; 2. Neuroscience Research Australia, Sydney, Australia. 3. St Vincent's Hospital Sydney Australia; 4. T Vincent's Applied Medical Research Center Sydney Australia
|
| |
|
 |
 |
|
|