 |
 |
 |
| |
CHARACTERIZATION OF HCV NS3 PROTEASE VARIANTS FROM PATIENTS ENROLLED IN A 28-DAY PHASE 2A TRIAL OF ACH-1625 DAILY DOSING PLUS PEG-IFN-ALPHA 2A/RBV
|
| |
| |
Reported by Jules Levin
European Association For The Study Of The Liver, Barcelona, Spain, April 18-22, 2012
Joanne Fabrycki, Dharaben Patel, Guangwei Yang, Yongsen Zhao, Steven Podos, Heather Robison, Lisa Robarge, Atul Agarwal, Elizabeth Olek, Milind Deshpande and Mingjun Huang
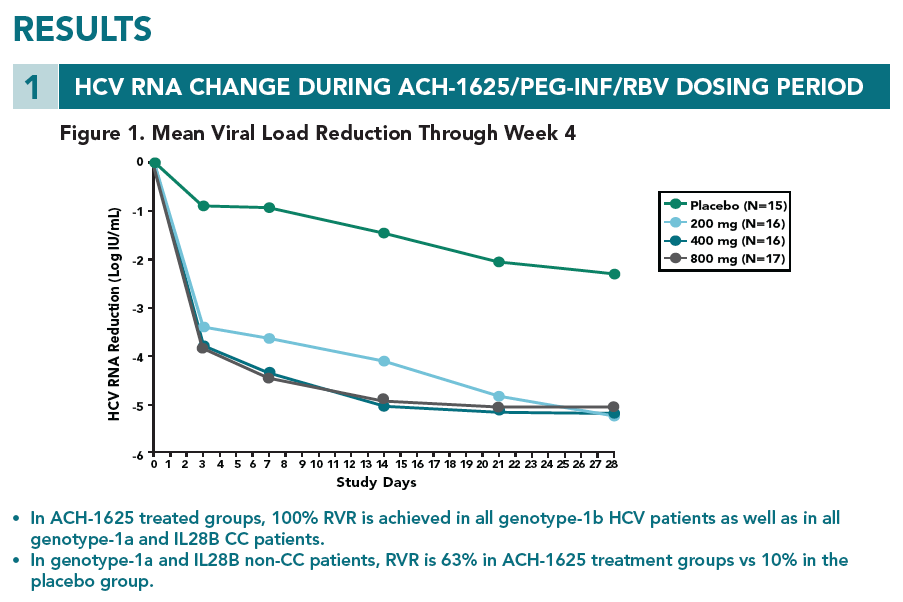
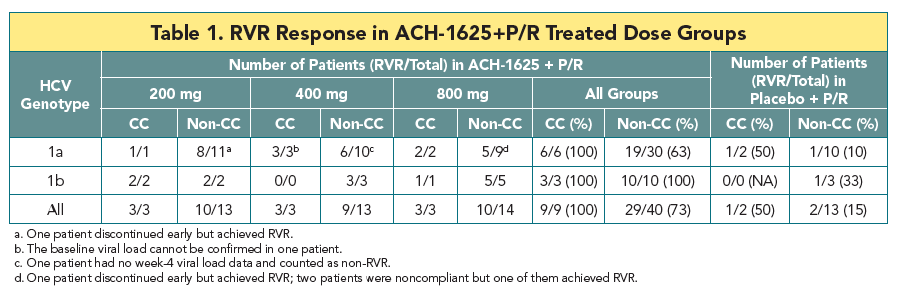
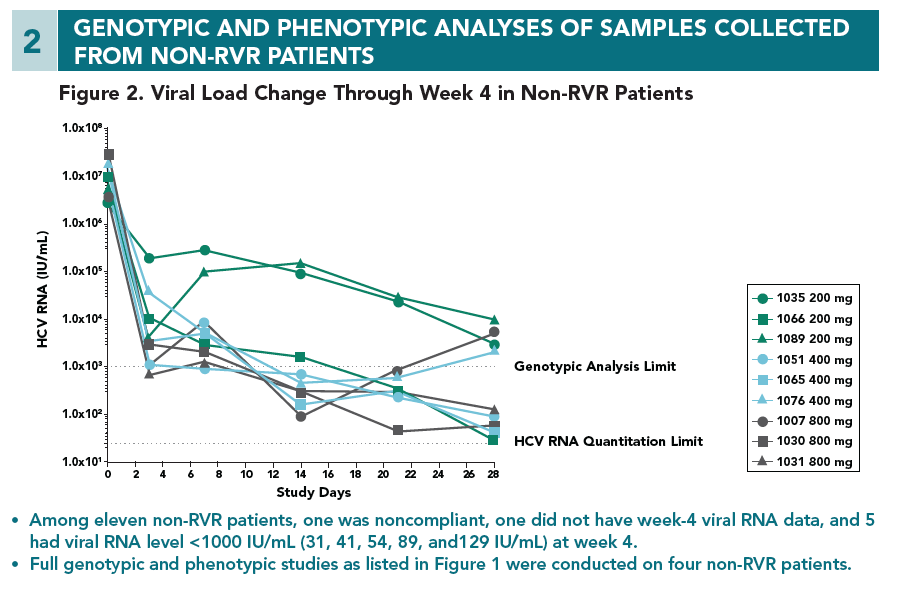
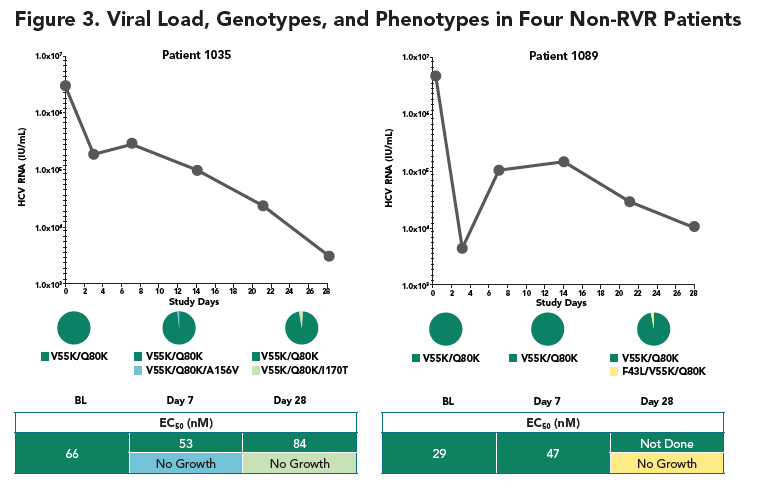
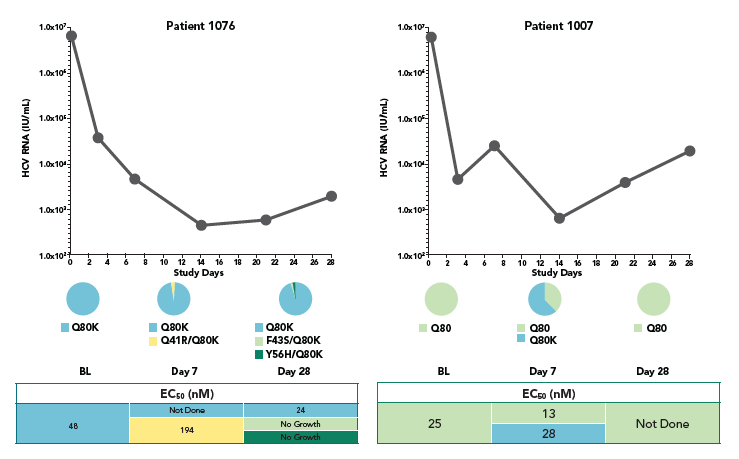
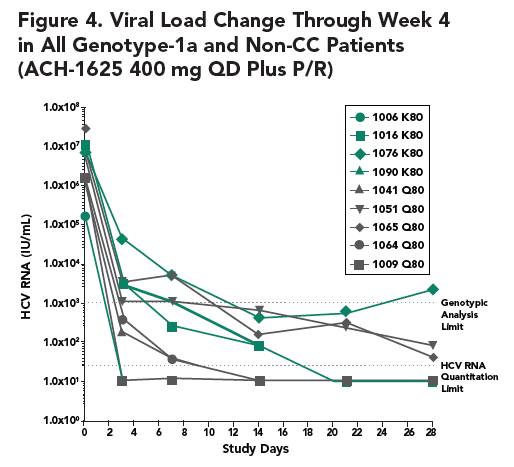
- At week 4, no mutations at the loci 155, 156, and 168 of NS3 that lead to high viral resistance to ACH-1625 were detected
- At week 1, an variant carrying a mutation at the locus 156 (A156V) was detected in one patient. The A156V mutation was present along with V55K and Q80K. This variant displayed poor growth fitness in vitro, consistent with its low frequency at week 1 and disappearance at week 4.
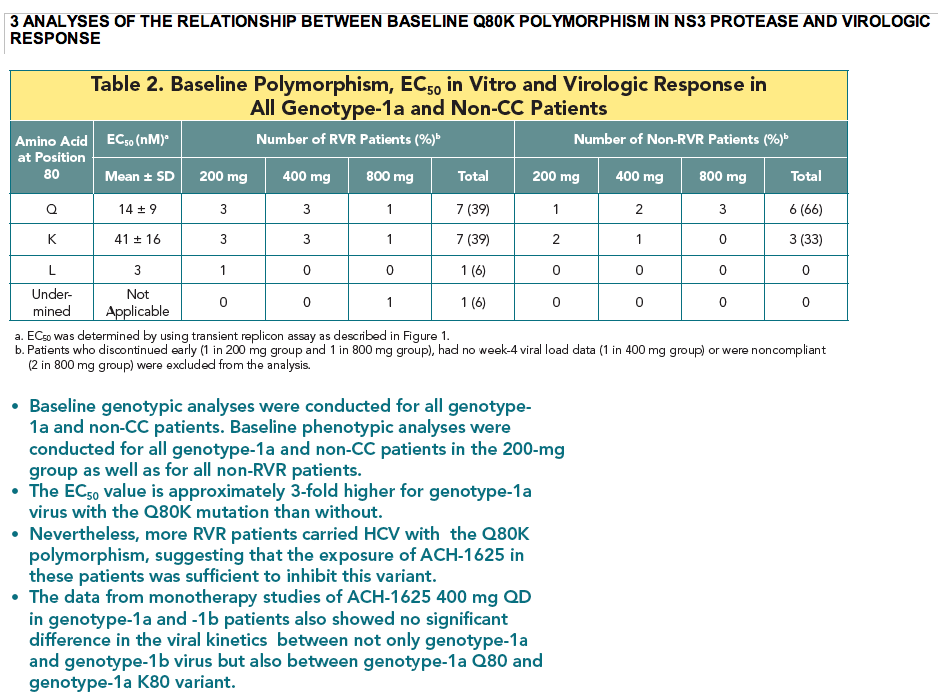
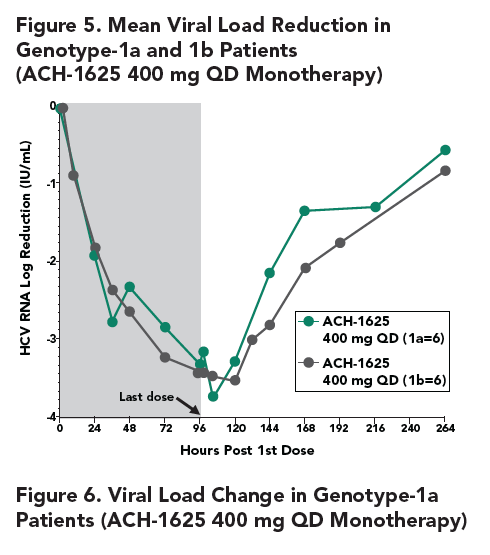
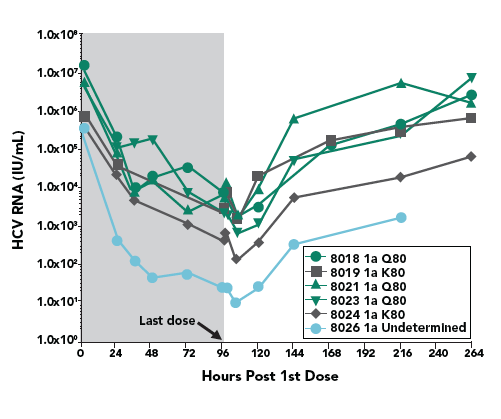
- The Q80K variant was detected in all 4 patients (3 at baseline and 1 during the treatment). To understand the role of Q80K polymorphism, further analyses were conducted as shown below.
|
| |
|
 |
 |
|
|