 |
 |
 |
| |
Long-Term Safety and Efficacy of Raltegravir (RAL)-Based VersusEfavirenz (EFV)-Based Combination Therapy in Treatment-Naïve HIV-1 Infected Patients:Final 5-Year Double-Blind Results from STARTMRK
|
| |
| |
Reported by Jules Levin
XIX International AIDS Conference
Washington, DC, USA
July 22-27, 2012
J. Rockstroh1, E. DeJesus2, M. Saag3, Y. Yazdanpanah4, J. Lennox5, H. Wan6, A. Rodgers6, M.J. DiNubile6, B-Y. Nguyen6, H. Teppler6, R. Leavitt6 and P. Sklar6 for the STARTMRK Study Team
1University of Bonn, Bonn, Germany; 2Orlando Immunology Center, Orlando, Florida; 3University of Alabama, Birmingham, Alabama; 4Hopital Bichat Claude Bernard, Paris, France; 5Emory University, Atlanta, Georgia; 6Merck Sharp & Dohme, Corp., Whitehouse Station, New Jersey
ABSTRACT
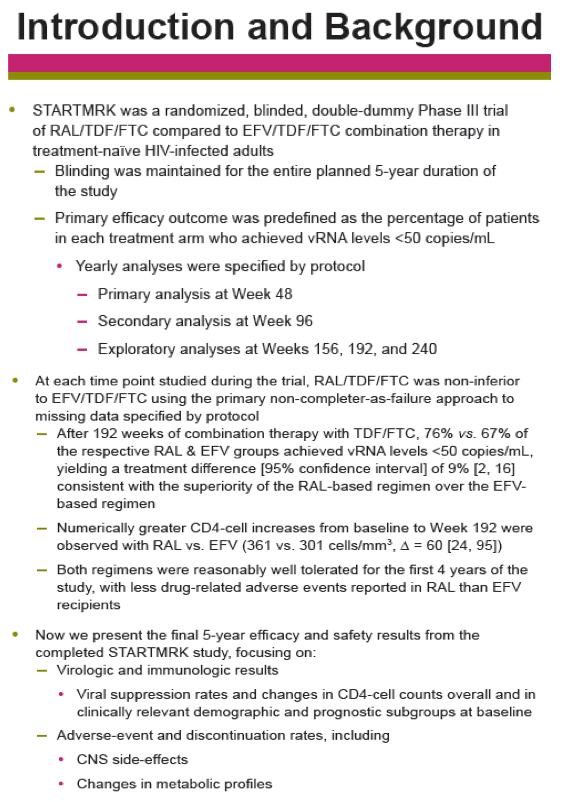
Background: As HIV treatment likely involves a lifelong commitment, long-term efficacy, & safety data are essential to selecting therapy. STARTMRK, a Phase III trial of RAL- vs. EFV-based therapy in treatment-naïve pts, remained blinded until its conclusion at 5 yrs. We now report the final study results.
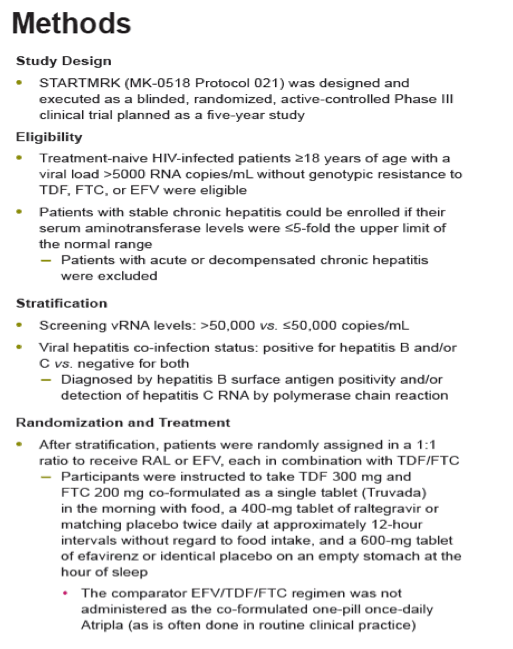
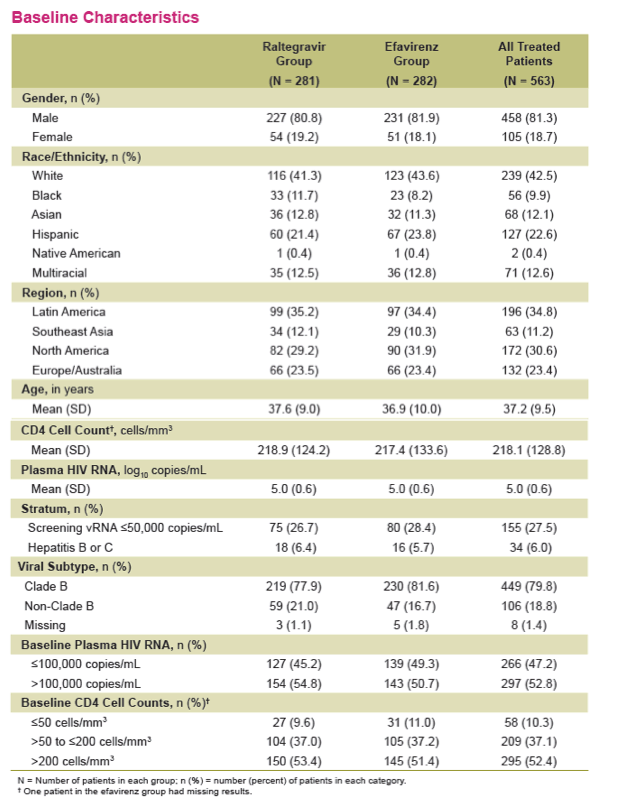
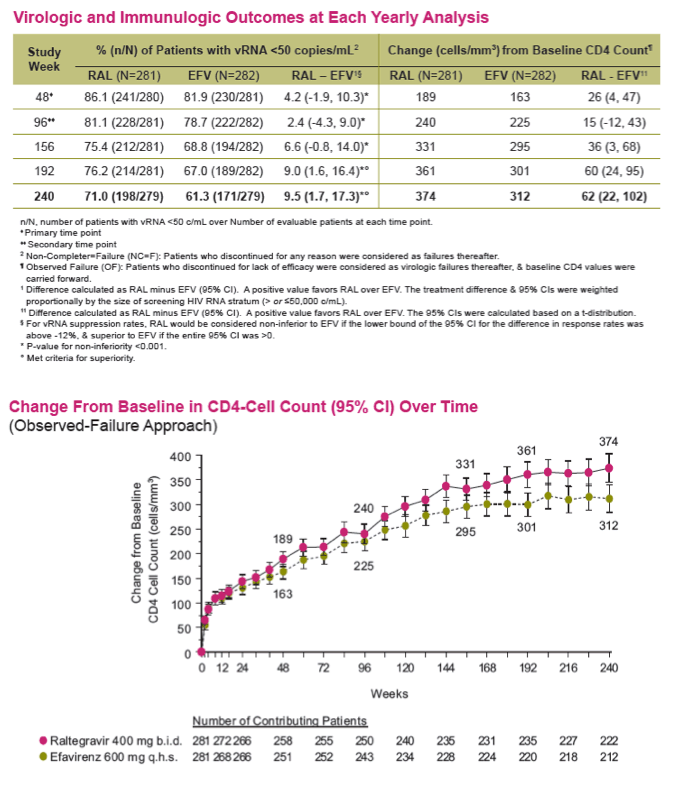
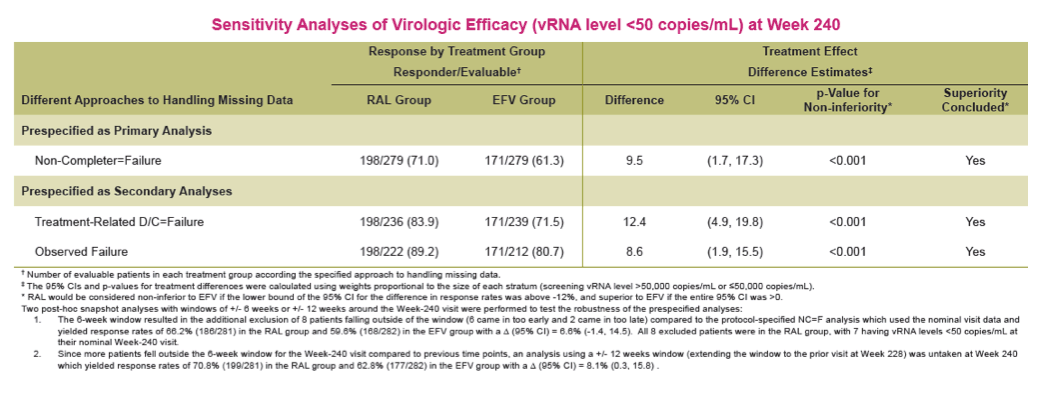
Methods: Previously untreated pts without baseline (BL) resistance to EFV, tenofovir (TDF) or emtricitabine (FTC) were eligible for a 5 yr, blinded, randomized study of TDF/FTC plus either RAL (400 mg bid) or EFV (600 mg qhs). Yearly analyses were planned, with primary & secondary endpoints stipulated at Wks 48 & 96. The primary efficacy outcome was the % of pts with vRNA levels <50 c/mL counting non-completers as failures. Changes from baseline CD4-count used an observed-failure approach to missing data. No formal hypotheses were formulated for testing at Week 240. An implicit fixed-sequence testing procedure was applied to control type I error given the multiple time points for testing non-inferiority; if non-inferiority were established, a subsequent test for superiority was specified in a closed testing fashion.
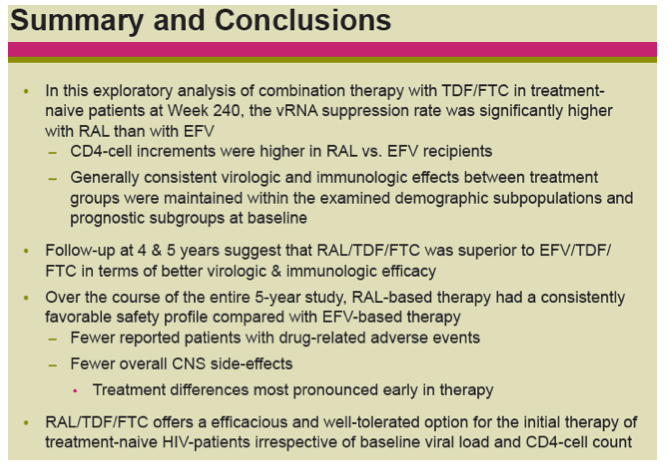
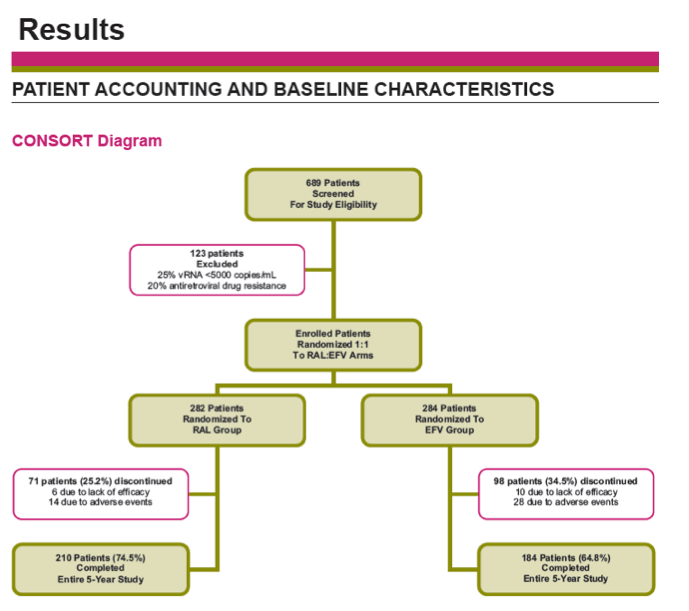
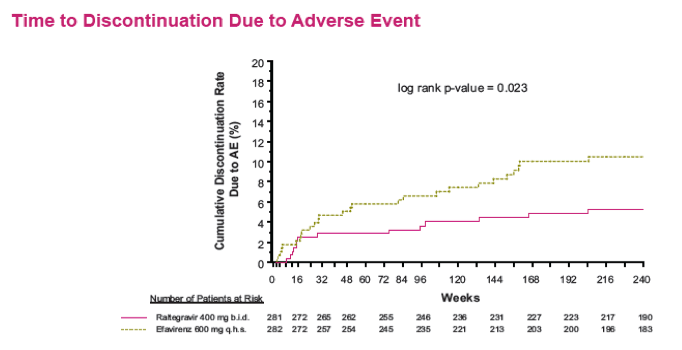
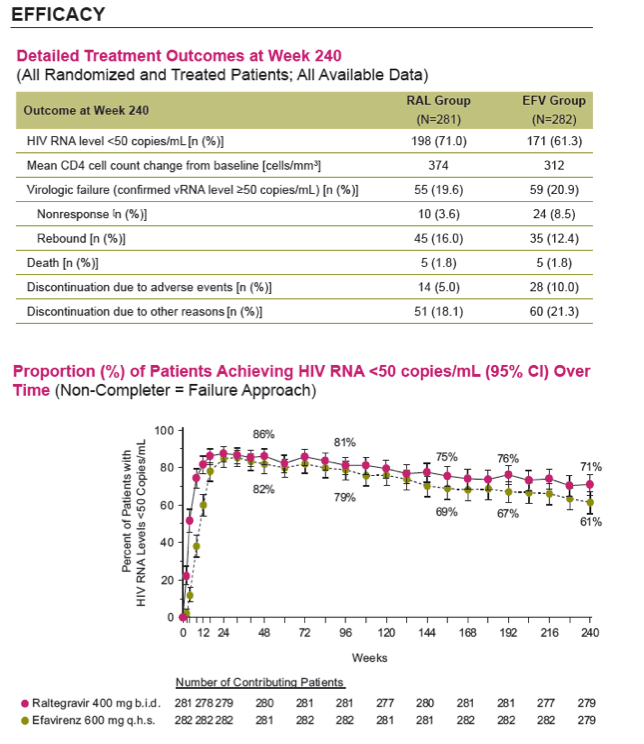
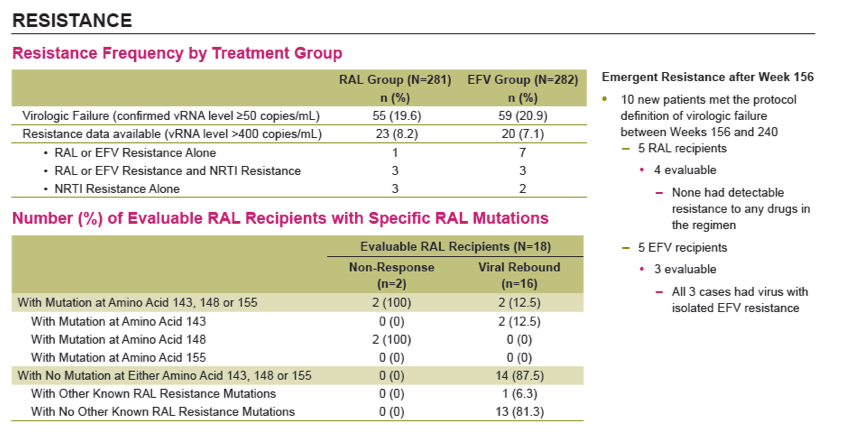
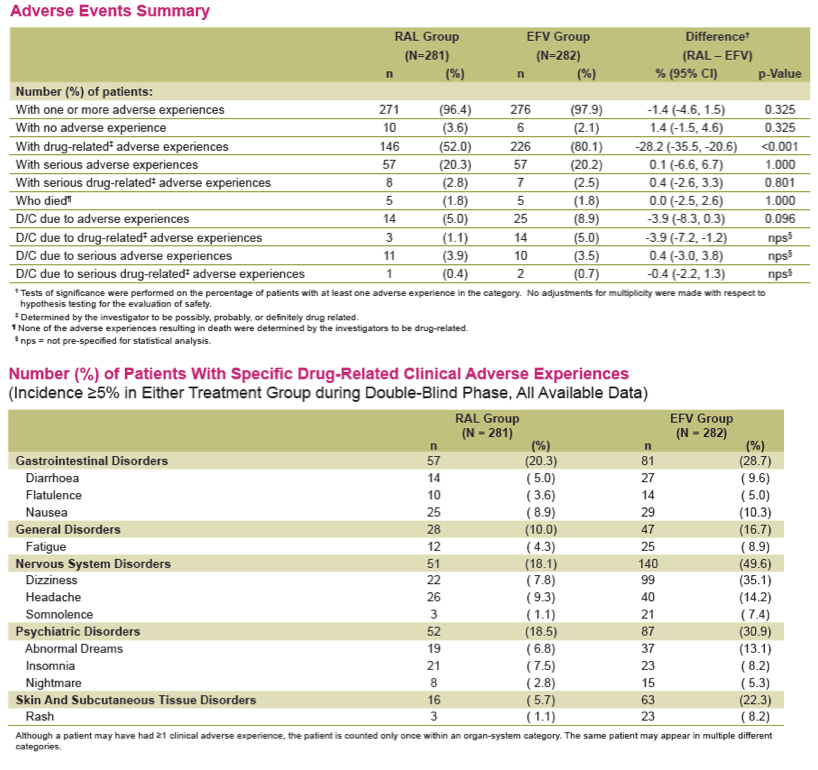
Results: Overall, 71/281 (25%) RAL & 98/282 (35%) EFV recipients discontinued the study; discontinuations due to clinical adverse events (CAE) occurred in 14 (5%) & 25 (9%) pts in the respective groups. Virologic results are tabulated below. Fewer pts with drug-related (DR) CAEs were reported among RAL (146, 52%) than EFV (226, 80%) recipients (nominal p<0.001).
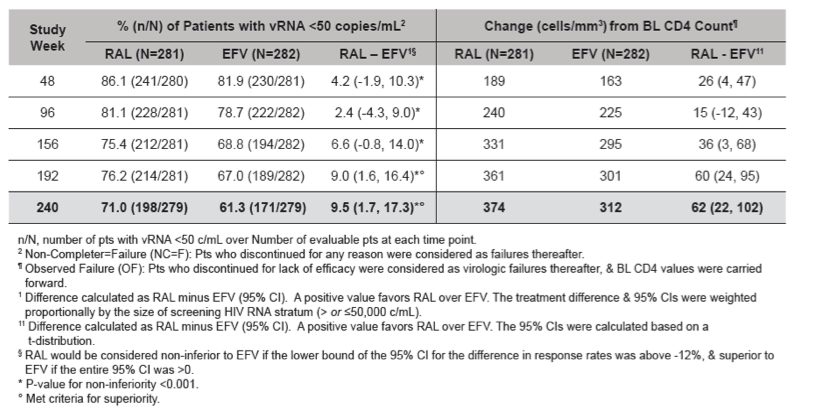
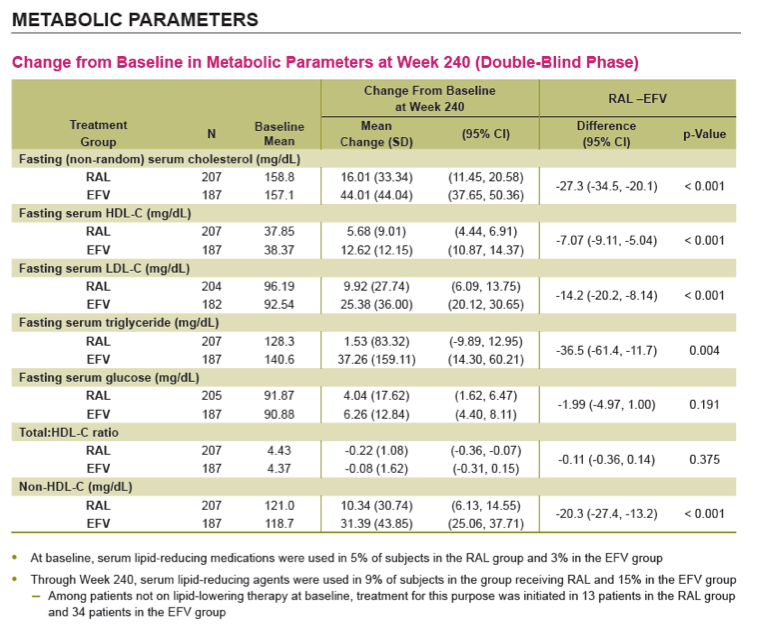
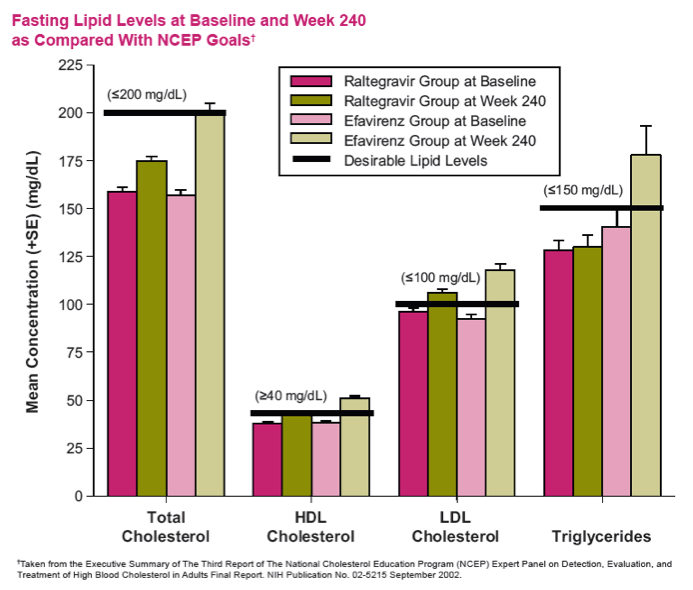
Conclusions: In this exploratory analysis of combination therapy with TDF/FTC in treatment-naive pts at Week 240, the vRNA suppression rate was significantly higher with RAL than with EFV. Follow-up at 4 & 5 yrs suggest that RAL/TDF/FTC was superior to EFV/TDF/FTC based on better virologic & immunologic efficacy. Over the entire 5-yr study, RAL-based therapy had a consistently favorable safety profile with less DRAEs compared with EFV-based therapy.



|
| |
|
 |
 |
|
|