 |
 |
 |
| |
2 New Studies at IAC Finding 'Functional Cure'
|
| |
| |
Reported by Jules Levin
"Berlin Patient, first person cured of HIV, may soon have company".
at bottom of report is abstract/link to webcast/slides for the 2 studies presented at conference that is subject of this report:
(study 1) transplant+HAART: 1st study oral presentation titled "Long-Term reduction in peripheral blood HIV-1 Reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation in 2 HIV+ individuals" --2 patients on HAART who underwent transplant, one patient had stage IV Hodgkins disease, he relapsed after standard treatment, recd transplant in 2008, 2nd patient was off ART for 20 yrs, diagnosed with had B-cell lymphoma, chemotherapy & HAART started, recd transplant in 2007, researchers studied reservoirs, PBMC HIV-1 DNA became undetectable 8 months after transplant. (study 2) the 2nd oral presentation of study is titled: "Distribution of the HIV Reservoir in patients spontaneously controlling HIV infection after treatment interruption"-- 11 patients recd HAART within 10 weeks of infection for average of 3 yrs & have stopped taking HAART for almost 7 yrs, since interruption these patients have long-term control of HIV after HAART interruption: controlled HIV with <1.7 log copies/ml, researchers looked at reservoirs and found low and inducible in resting CD4 subsets. Joe Eron commented these patients could have been elite controllers without needing HAART but researcher explained why she did not think they are not just like elite controllers, that the results appear to be as a result of early HAART. Steve Deeks commented early HAART may be key to this finding.
"Scientists make curing HIV a priority"
Once seen as impossible, a cure is now viewed as a realistic goal by more and more researchers, who argue the epidemic cannot be contained through treatment and prevention alone.
(1) "two HIV-positive men from Boston who developed lymphoma. In both cases, their treatment included a bone marrow transplant, which results in a new immune system. The bone marrow donors did not have HIV.......The patients were conditioned for their transplants....with HAART"
(2) "Another study presented Thursday reported on 12 French HIV-positive patients who received early treatment and have acquired an ability to naturally control HIV. These patients offer hope that it may be possible to "functionally" cure the disease"
"EARLY HAART: The results provide further evidence that antiretroviral therapy should be started soon after infection, researchers said."
"To wipe out all traces of HIV, scientists must find a way to wake up reservoirs of sleeping virus and target them with drugs"
"Deeks and Barre-Sinoussi estimated that governments and foundations invested about $75 million in HIV cure research in 2011, but said the effort would require hundreds of millions of dollars in annual funding until a cure is found"
-----------------------------
Berlin Patient, first person cured of HIV, may soon have company
By Erin Loury Los Angeles Times
July 27, 2012, 2:26 p.m.
Washington, D.C.-
The Berlin Patient, the only person considered cured of HIV, may soon have some company.
Researchers at the International AIDS Conference in Washington, D.C., made presentations Thursday on two HIV-positive men from Boston who developed lymphoma. In both cases, their treatment included a bone marrow transplant, which results in a new immune system. The bone marrow donors did not have HIV.
The patients were conditioned for their transplants with a reduced-intensity protocol that allowed them to maintain enough strength to continue taking antiretroviral drugs to keep their HIV in check. These drugs are usually too toxic for HIV-positive cancer patients to handle.
So far, it appears that their new immune systems have remained HIV-free. Seventeen months after the transplants, researchers could not detect any HIV genetic material in the patients' blood. They say the credit for this goes to the antiretroviral drugs the patients are taking.
Still unclear is whether the virus still lurks in the patients' tissues. "It is possible that there is still other residual HIV material, "said study author Dr. Timothy Henrich of Harvard University and Brigham and Women's Hospital. If doctors became convinced that all trace of the virus is gone, the patients could stop taking the antiretroviral drugs and be considered cured.
But they're not there yet.
"We're being very carful to refer to our patients as not being functionally cured," said study author Daniel Kuritzkes, also of Harvard University and Brigham and Women's Hospital. Only when these patients can successfully stop their medication can they be considered cured of HIV.
The only person in that category right now is the Berlin Patient, a.k.a. Timothy Brown. He had a bone marrow transplant to treat acute myeloid leukemia. In his case, the bone marrow donor was not only HIV-negative, but had a rare genetic mutation that blocks HIV from entering cells. That effectively makes Brown immune to the virus, and his body has remained HIV-free even without taking antiretroviral drugs.
The two patients in Boston received their bone marrow transplants from people who did not have the rare genetic variant, which is why they are still taking their drugs.
Researchers are working on potential cures that involve transferring that genetic mutation to HIV-positive patients without taking on the significant risks of a bone marrow transplant.
Another study presented Thursday reported on 12 French HIV-positive patients who received early treatment and have acquired an ability to naturally control HIV. These patients offer hope that it may be possible to "functionally" cure the disease - enabling people to tolerate HIV without completing ridding their bodies of the virus.
The patients started taking antiretroviral medication within 10 weeks of their HIV infections and stopped about three years later. Now, nearly seven years after ending treatment, these patients still have very low levels of the virus in their bodies. They appear similar to a small group of people known as "elite controllers" who naturally suppress HIV to low levels and do not get sick from the virus.
Reservoirs of dormant HIV can occur throughout the body. This sleeping HIV can periodically wake up and re-infect a person, which is why people with HIV usually have to take medication for life.
The French patients still have very low reservoirs of virus in their bodies, and researchers wondered if their ability to manage the virus related to how these remaining patches of HIV were distributed among a type of white blood cells called memory T cells. Pockets of HIV usually build up in long-lived types of memory T cells. However, researchers found that this rare cohort had dormant HIV primarily in shorter-lived memory T cells - similar to elite controllers - so their infected cells don't persist as long.
The results provide further evidence that antiretroviral therapy should be started soon after infection, researchers said.
To wipe out all traces of HIV, scientists must find a way to wake up reservoirs of sleeping virus and target them with drugs. Jerome Zack, director of the UCLA Center for AIDS Research, made a presentation about this approach.
Zack and his colleagues outfitted little lipid bubbles called liposomes with antibodies that specifically match up with CD4 cells, the type of white blood cell that HIV usually attacks. The liposomes deliver two drugs to the cell - one, called bryostatin, switches on cell activity, and the other, a protease inhibitor, prevents HIV virus from assembling more virus once it starts to reproduce.
These liposomes didn't target or otherwise activate another type of white blood cells called CD8 cells. As a result, the treatment didn't trigger toxic inflammation. However, scientists still need to figure out how to design liposomes that identify only the CD4 cells that are infected with dormant HIV.
Bryostatin is a useful molecule, but also exorbitantly expensive - a single gram can cost $1 million.
Zack and his colleagues published a paper in Nature Medicine last week that described a number of synthetic products that mimic bryostatin, and cost only about $2,000 per gram to manufacture. Zack said that a few of these compounds also outperform their natural counterpart in switching on latent HIV while triggering less inflammation.
--------------------------------
"Scientists make curing HIV a priority"
By Erin Loury Los Angeles Times
July 27, 2012, 2:26 p.m.
Washington, D.C.-
An influential group of scientists gathered this week at the International AIDS Conference in Washington, D.C., is committing to a goal that just five years ago would have seemed ludicrous: to cure HIV.
After studying the virus for more than 30 years and developing potent drugs that transformed the disease from a death sentence into a manageable chronic condition, a growing number of researchers now say the search for a cure should be a major research priority. While acknowledging substantial challenges, they argue that the effort is necessary because the epidemic cannot be contained through treatment and prevention alone. And recent medical and scientific advances - including the case of the first man definitively cured of HIV - offer proof that it's possible.
Spearheading this audacious challenge is the International AIDS Society, which developed a research agenda in collaboration with more than 40 scientists led by French virologist Francoise Barre-Sinoussi, who won the Nobel Prize in physiology or medicine in 2008 for her role in the discovery of HIV. Two years in the making, the scientific strategy provides a road map for moving research for a cure forward. Among the tasks: investigating where and how the virus can hide out in the body and studying the immune response of the select group of people who are naturally immune to HIV.
Developing drugs that keep HIV in check has so far proved more feasible than trying to eradicate it, said Dr. Steven Deeks, a member of the AIDS Research Institute at UC San Francisco. But now that more than 20 antiretroviral therapies can prolong the lives of people with HIV for decades, he said, it's time to aim higher.
"I think these drugs have gotten as good as they're going to get," said Deeks, who worked with Barre-Sinoussi to develop the research plan. "We need to shift from blocking the virus from replicating to essentially getting rid of the virus."
The antiretroviral drugs are lifesaving, but they have problems. Treatment is toxic and expensive, and only about half of the world's 34 million people living with HIV who need the drugs can get them. Patients must take the drugs daily for the rest of their lives to keep the virus at bay.
"It's just practically difficult to treat people all their life with therapy, even if it's very simple therapy," said Dr. David Margolis, director of the Program in Translational Clinical Research at the University of North Carolina at Chapel Hill.
But for a long time, there has been no alternative. HIV researchers suffered a major setback in the 1990s when they discovered that the strongest drug cocktails could substantially knock down a patient's viral load but couldn't wipe it out completely. If people stopped taking medication, the disease came roaring back.
Pessimism about finding a cure set in, said Paula Cannon, a molecular biologist at USC's Keck School of Medicine. Even five years ago, a scientist who proposed HIV cure research "would be laughed out of the room," she said. "Nobody would give you money."
What changed? "It's come down to one man," Cannon said.
That man is Timothy Brown, known to the medical world as the Berlin Patient.
Brown was an HIV-positive American who was living in Germany when he developed leukemia. After failing to respond to first-line cancer treatments, he opted for a bone marrow transplant in 2007. As his doctors searched for a suitable donor, they looked for one with a rare genetic mutation that disables a receptor known as CCR5, which HIV needs to gain entry into immune cells. Brown had two transplants that not only put his leukemia into remission but replaced his HIV-susceptible immune system with one that could ward off the disease.
Brown no longer takes antiretroviral drugs and no longer tests positive for HIV. Essentially, he is cured.
"There's nothing like success to galvanize the research," Cannon said. "People are daring to hope again that with a lot of hard work and ingenuity, scientists can deliver."
Bone marrow transplants aren't suitable for widespread use: The procedure Brown received ends in death 20% of the time, and finding an appropriate donor would be a long shot in most cases. So scientists are working on alternatives.
There are two general approaches. One, an elimination cure, would rid the body of all HIV-infected cells. The other, a functional cure, would engineer a patient's own immune system to resist HIV, even if the virus remains present in the body.
For an elimination cure to work, researchers must learn to identify the dormant HIV that hides in immune cells and tissues, evading assault from drugs. Much of the International AIDS Society's plan focuses on this problem.
Efforts to flush the virus from its hiding places are showing signs of progress. For instance, Margolis and his colleagues have found that giving patients a drug called a histone deacetylase inhibitor can prompt HIV to wake up and start producing proteins. Drugs and the immune system can then recognize those proteins and mount an attack. Margolis presented these results at a research conference in March.
But it's far from clear how long this activation would last, or how to empty all of the hiding places. An effective elimination cure would also have to root out every last speck of HIV, including those in hard-to-reach areas like the brain and spleen.
One approach to a functional cure involves using gene therapy to modify a patient's DNA so that it produces immune cells with a disabled CCR5 gene. In theory, the result would be the same as with the Berlin Patient, said Dr. Jay Levy, who co-discovered the AIDS virus in 1983 and directs the Laboratory for Tumor and AIDS Virus Research at UCSF.
There are various hurdles, including finding ways to safely knock out the CCR5 gene.
"It's going to be like going to the moon again, but it's so important that we do this," said Cannon, who is working on a gene-therapy project that could be ready for clinical trials in two years.
Dr. Bernhard Schwartlander, a director with the Joint United Nations Program on HIV/AIDS, said the recent scientific developments make an HIV cure an exciting prospect. "It's the right point in time to actually make that a special topic in the AIDS response," he said. "If you don't focus on [a cure] now ... you will never get there."
It won't come cheap. Writing last week in Nature, Deeks and Barre-Sinoussi estimated that governments and foundations invested about $75 million in HIV cure research in 2011, but said the effort would require hundreds of millions of dollars in annual funding until a cure is found.
Dr. Anthony Fauci, director of the National Institute for Allergy and Infectious Diseases, called the search for an HIV cure an "aspirational goal." He emphasized that finding a cure is not necessarily synonymous with turning the tide on the AIDS epidemic.
"You can end the AIDS pandemic without necessarily curing anybody," by preventing new transmission and treating those with HIV, he said. "Or you can cure a small number of people and still have the epidemic be raging."
Levy acknowledged that even if a cure were discovered, it could take years to become practical in low- and middle-income countries, where 97% of the people with HIV live. But right now, he said, finding a cure is like "the four-minute mile - what we need to do is just show it's possible" - and after that, "there's enough creativity out there to find a way of having it applied in all parts of the world."
-------------------------------
Long-term reduction in peripheral blood HIV-1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation in two HIV-positive individuals
Presented by Timothy J. Henrich (United States).
14:30
THAA0101
Abstract
Powerpoint
Webcast
T.J. Henrich1,2, G. Sciaranghella3, J.Z. Li1,2, S. Gallien4, V. Ho2,5, A.S. LaCasce2,5, D.R. Kuritzkes1,2
1Brigham and Women's Hospital, Boston, United States, 2Harvard Medical School, Boston, United States, 3Ragon Institute of MGH, MIT and Harvard, Boston, United States, 4Hopital Saint-Louis, Paris, France, 5Dana-Farber Cancer Institute, Boston, United States
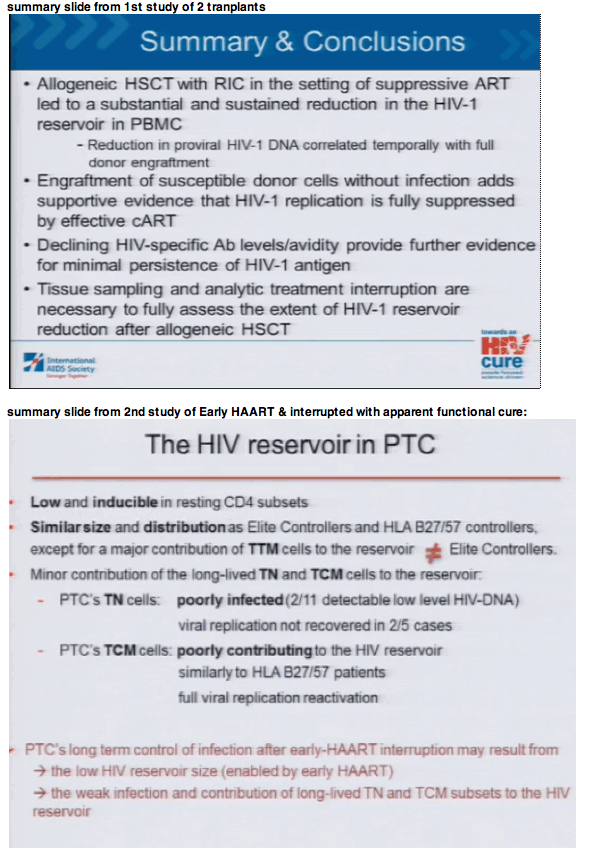
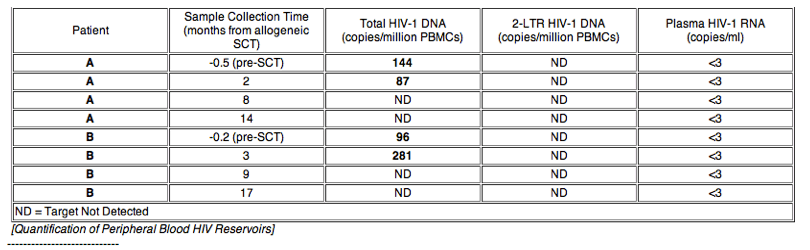
Background: Functional HIV-1 cure has been described in the setting of myeloablative allogeneic stem cell transplant (alloSCT) with ccr5∼32/ ccr5∼32 donor cells, but the effects of alloSCT on viral reservoirs are largely unknown. We studied the longitudinal effects of reduced-intensity conditioning (RIC) alloSCT on HIV-1 peripheral blood reservoirs in two infected male patients with hematologic malignancies who previously underwent autologous SCT.
Methods: Analysis of peripheral HIV-1 reservoirs was performed on banked samples (1 pre- and 3 post-RIC-AlloSCT) for both patients, including: 1) quantification of HIV-1 DNA from peripheral blood mononuclear cells (PBMCs), 2) quantification of 2-LTR circles from PBMC episomal DNA, 3) full-length envelope amplification and phenotypic coreceptor usage prediction from proviral DNA, 4) quantification of plasma viremia by a single-copy assay, 5) flow cytometric characterization of lymphocyte subsets and coreceptor expression, and 6) CCR5 genotyping.
Results: No HIV-1 DNA was detected 8 to 17 months after alloSCT in PBMC from both patients despite presence of modest levels of total PBMC-associated HIV-1 DNA prior to and 2-3 months after SCT (87-271 copies/106 PBMCs). 2-LTR circles were not detected at any time-point despite excellent recovery of episomal mitochondrial DNA. Both patients were heterozygous for ccr5∼32 mutation prior to transplant; a transient reduction in CXCR4 expression was observed following transplant. Pseudoviruses incorporating envelopes from early time-points used predominately CCR5 for entry. Both patients remained virologically suppressed on ART, but were either started on prednisone or continued on tacrolimus/sirolimus immunosuppressive therapy for chronic graft-versus-host disease (GVHD) near the time of loss of HIV-1 reservoir detection.
Conclusions: PBMC HIV-1 DNA became undetectable 8 months after RIC-alloSCT. This finding may be due to a dilutional effect of donor cell engraftment in the setting of protective ART, the additive effect of cytotoxic therapies, and/or GVHD. Confirmation of results by sampling large-volume blood collections and other tissue compartments is warranted.
----------------------------
Distribution of the HIV reservoir in patients spontaneously controlling HIV infection after treatment interruption
Presented by Charline Bacchus (France).
15:00
THAA0103
Abstract
Webcast
C. Bacchus1, L. Hocqueloux2, V. Avettand-Fenoel3, A. Saez-Cirion4, A. Melard3, B. Descours5, A. Samri1, C. Blanc6, B. Autran1, C. Rouzioux3, VISCONTI and ALT ANRS study groups
1Cellular and Tissular Immunology Laboratory, Pierre and Marie Curie University, INSERM UMR-S 945, Pitie-Salpetriere Hospital, Paris, France, 2Infectious and Tropical Diseases Department, Regional Hospital, Orleans, France, 3Virology Laboratory, Rene Descartes University, Necker Hospital, Paris, France, 4Institut Pasteur, Unite de Regulation des Infections Retrovirales, Paris, France, 5Human Genetic Institute, Molecular Virology Laboratory, CNRS UPR1142, Montpellier, France, 6Flow Cytometry Platform CyPS, Pierre and Marie Curie University, Pitie-Salpetriere Hospital, Paris, France
Background: Virological and Immunological Studies in CONtrollers after Treatment Interruption (VISCONTI) are required to understand the benefits of an early treatment at acute HIV-1 infection on the HIV reservoir. We studied the distribution, magnitude and inducibility of the HIV reservoir in VISCONTI patients.
Methods: The prospective VISCONTI study included twelve patients controlling HIV for a median of 76[IQR:67.5-84.5] months after interruption of a 3[IQR:1.7-5.9] years long HAART initiated within 10 weeks post-infection. Circulating resting CD25-CD69-HLADR- CD4+T cell subsets were sorted as naive (TN), central-memory (TCM), transitional-memory (TTM) and effector-memory cells (TEM) for further cell-associated HIV-DNA quantification by ultrasensitive real-time-PCR, and viral inducibility by culture with anti-CD3/anti-CD28/IL-2/IL-7. Reservoir distribution was compared to the one observed in 8 untreated Elite-Controllers for whom 90% of HIV-RNA measures was undetectable (below 200 copies) over 12[9-14] years.
Results: In the VISCONTI group, activated CD4+T cells had significantly higher HIV-DNA levels than resting ones (median 2.7[IQR:2.4-3.4] and 2[IQR:1.8-2.5] log copies/million cells, p=0.005). HIV-DNA was detected in all subsets from all patients except for 8 out of 12 TN-sorted cells, which were 10 fold less infected than all memory subsets (median TN:1.5[IQR:1.2-1.6], TCM:2.5[IQR:1.8-2.9], TTM:2.6[IQR:2.2-2.8] and TEM:2.4[IQR:2-2.8] log copies/million cells, p< 0.007). TTM was the major subset contributing to 56% of this reservoir. The same HIV reservoir characteristics were observed in Elite-Controllers in term of magnitude and distribution, except that both TCM and TTM equally contributed to the Elite-Controllers HIV reservoir. The VISCONTI HIV reservoir was inducible after TCR-stimulation in all sorted memory subsets from all patients, except in TN where no virus was recovered in 6 out of 8 patients.
Conclusions: In VISCONTI patients, treatment initiated at primary HIV-1 infection leads, after treatment interruption, to a low -but inducible- durable HIV reservoir distributed mainly in short-lived memory CD4+T cells that mimicks the natural distribution observed in Elite-Controllers.
|
| |
|
 |
 |
|
|