 |
 |
 |
| |
Dolutegravir (DTG; S/GSK1349572) + Abacavir/Lamivudine Once Daily Statistically Superior to Tenofovir/Emtricitabine/Efavirenz: 48-Week Results - SINGLE (ING114467)
|
| |
| |
Reported by Jules Levin
52nd ICAAC Sept 9-12 San Francisco
S. Walmsley, MD - Professor of Medicine1, A. Antela, MD - Doctor 2, N. Clumeck, MD - Professor 3, D. Duiculescu, MD - Doctor 4, A. Eberhard, MD - Doctor 5, F. Gutierrez, MD - Doctor 6, L. Hocqueloux, MD - Doctor 7, F. Maggiolo, MD - Doctor 8, U. Sandkovsky, MD - Asst. Professor of Medicine 9, C. Granier, DESS - Mgr, Statistics 10, B. Wynne, MD - Physician Project Leader 10, K. Pappa, PharmD - Clinical Investigation Leader 10;
1Univ Hlth. Network, Toronto, Canada, 2Hosp. Clinico Univ, Santiago de Compostela, Spain, 3Ctr Hosp Univ Saint-Pierre, Brussels, Belgium, 4Infectious Tropical Diseases Hosp Dr. Victor Babes, Bucharest, Romania, 5MVZ Karlsplatz HIV Res/Clin Care Ctr, Munich, Germany, 6Hosp Univ de Elche, Alicante, Spain, 7Ctr Hosp Regional d'Orleans, Orleans, France, 8Antiviral Therapy Unit Ospedali Riuniti, Bergamo, Italy, 9Univ Nebraska Med Ctr, Omaha, NE, 10GlaxoSmith-Kline, RTP, NC.
ABSTRACT
Background: Dolutegravir (DTG; S/GSK1349572), a once-daily, unboosted integrase inhibitor, has shown rapid & durable antiviral responses, with favorable tolerability.
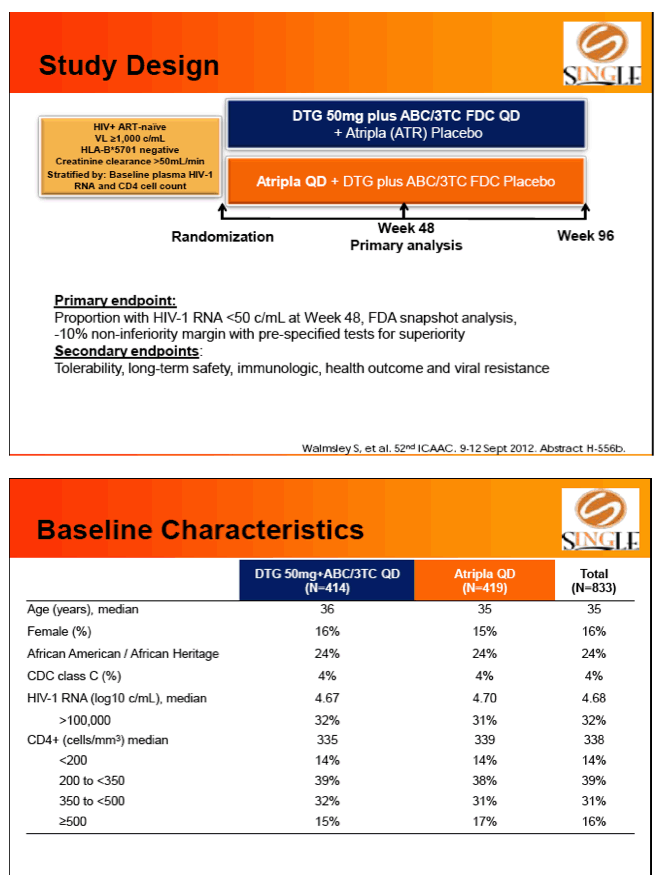
Methods: SINGLE, a double-blind, double-dummy, non-inferiority phase III study in therapy-naïve adults with HIV-1 RNA ≥1000 c/mL, randomized subjs to DTG 50 mg + ABC/3TC QD or TDF/FTC/EFV QD.
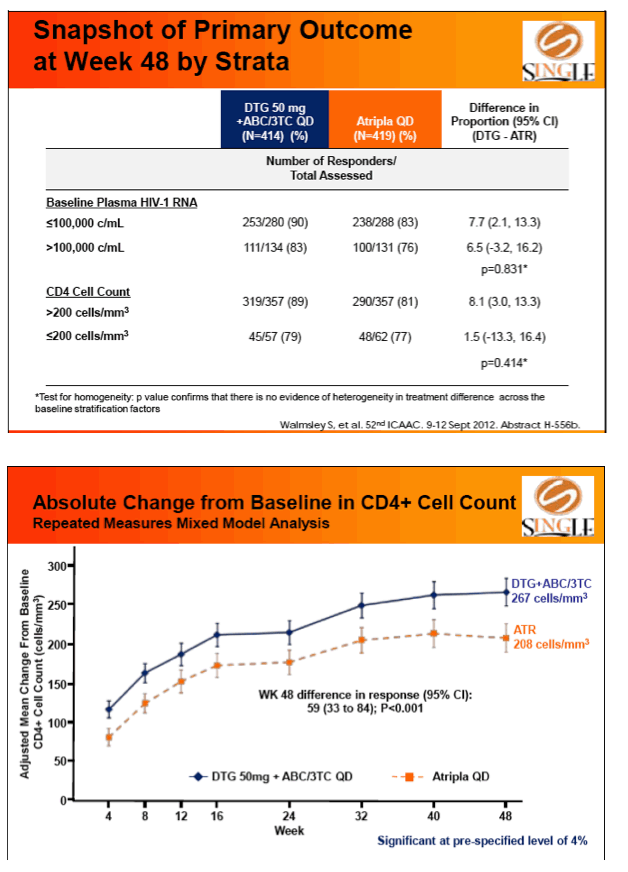
Primary endpoint: proportion of subjs with HIV-1 RNA <50 c/mL at week 48 (FDA Snapshot, ITT-Exposed). Tolerability, safety, & viral resistance evaluated.
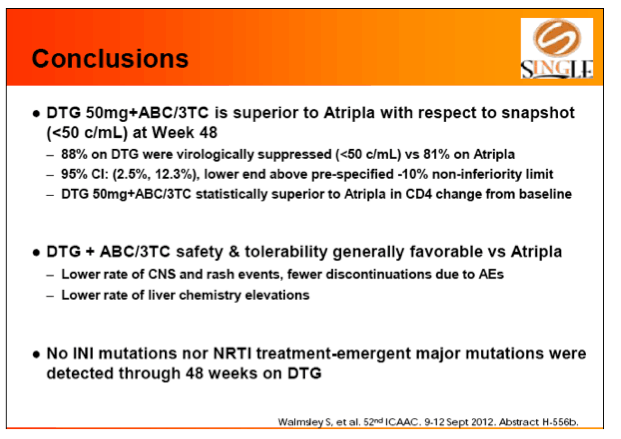
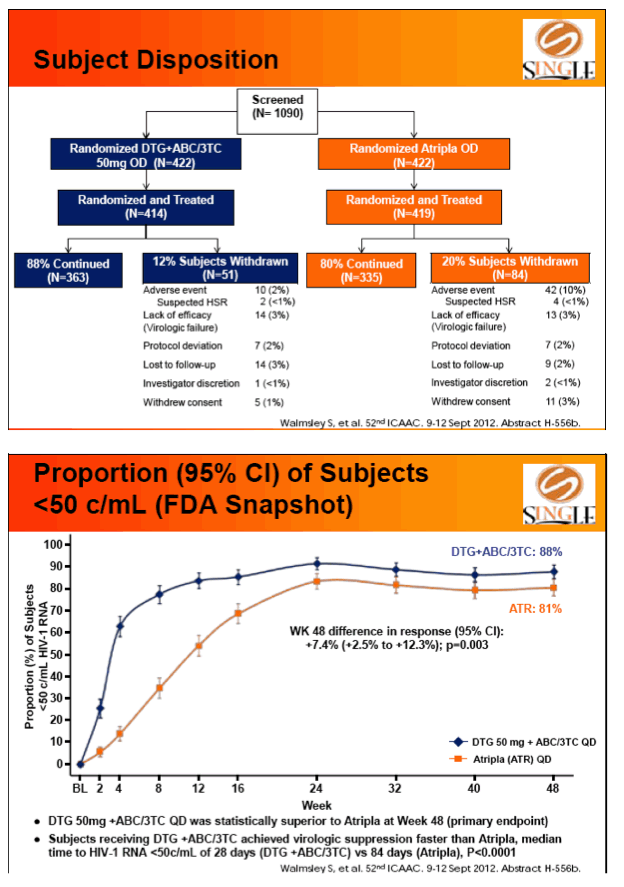
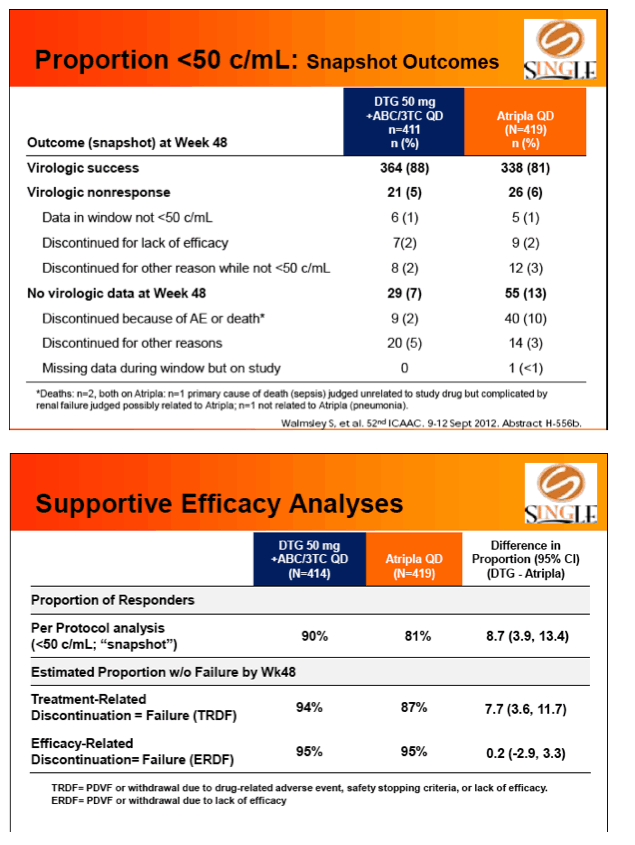
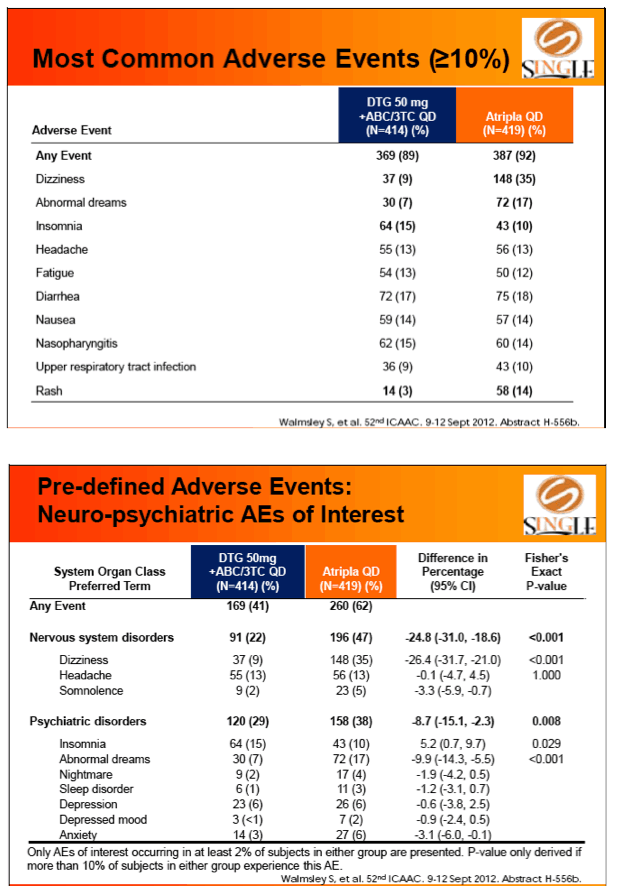
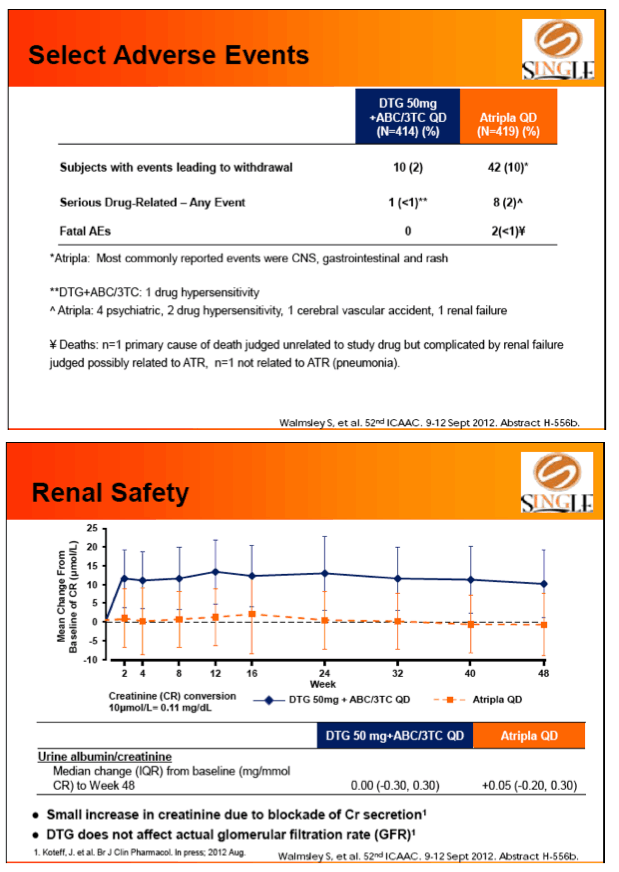
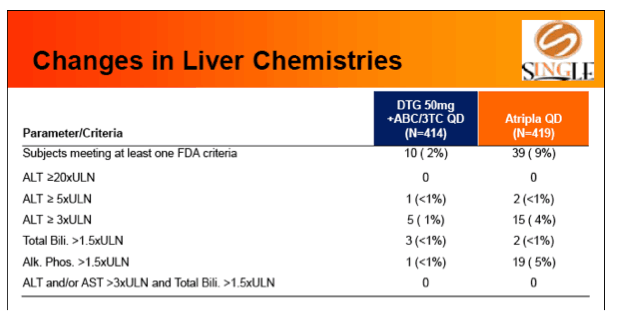
Results: 833 enrolled (84% males; 32% non-white); groups similar at BL. At Week 48, 88% of DTG + ABC/3TC and 81% of TDF/FTC/EFV subjects had HIV-1 RNA <50 c/mL confirming non-inferiority. Using pre-specified testing procedure, statistical superiority was concluded (p=0.003). Both Time to Suppression and Change from BL in CD4 favored DTG arm and were significant. Protocol defined virological failure: 4% on each arm. No INI or NRTI resistance observed on DTG arm, vs. 1 NRTI RAM and 4 NNRTI RAMs on TDF/FTC/EFV.
Conclusion: DTG + ABC/3TC was highly effective and better tolerated through 48 weeks than TDF/FTC/EFV single-tablet regimen.


|
| |
|
 |
 |
|
|