 |
 |
 |
| |
Atazanavir (ATV) and Darunavir (DRV) Cristalluria and High ATV and DRV Concentrations in Urine of Asymptomatic Patients Receiving ATV and DRV Based Regimens
|
| |
| |
Reported by Jules Levin
52nd ICAAC Sept 9-12 2012 SF
Victoire de Lastours1, Erika Silva1, Michel Daudon2, Raphael Porcher3, Hélène Sauvageon4, Jean-Michel Molina1
1. Infectious Diseases Dept, St Louis Hospital, APHP and Sorbonne Paris Cité, University Paris 7, Paris, France ; 2. Biochemistry Dept, Tenon Hospital, University Paris 6, Paris, France.; 3.Biostatistics Dept. St Louis Hospital, APHP, Paris, France ; 4. Pharmacology Dept, St Louis Hospital, APHP, Paris, France
CONCLUSIONS
· Atazanavir and Darunavir concentrate highly in the urine of asymptomatic patients, which is not the case for Lopinavir.
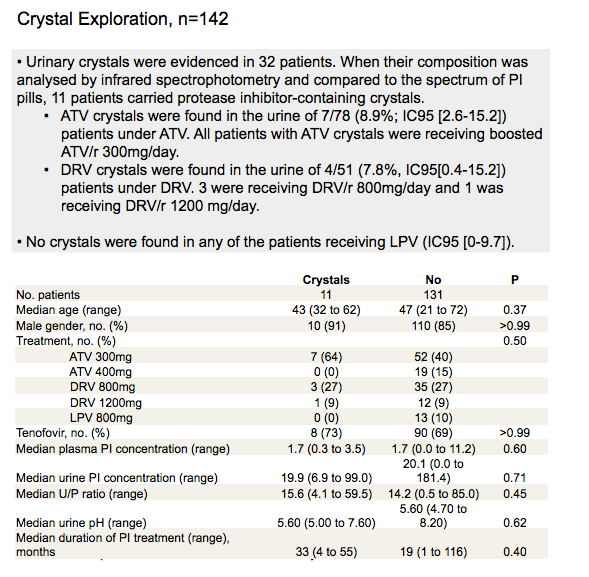
· ATV-crystals but also DRV-crystals (which have never yet been described) were evidenced in the urine of a few asymptomatic patients receiving ATV and DRV-based regimen. No lopinavir crystals were evidenced. Attention should be paid towards the potential renal toxicity of DRV as well as ATV.
· No risk-factor associated with the presence of urinary PI crystals could be evidenced, probably due to the small study size.
· Further studies need to confirm these findings and determine if there is a correlation between the presence of crystals and the occurrence urolithiasis.
ABSTRACT
Background: Atazanavir (ATV), an HIV protease inhibitor (PI), has been associated with kidney stones and renal failure. In order to understand why ATV and not other PIs are responsible for these events, we measured urine and plasma concentrations of recent PIs and searched for PI-crystals in the urine of asymptomatic patients.
Methods: Patients on stable antiretroviral including boosted 300mg/d ATV (ATV300/r), unboosted ATV 400mg/d (ATV400), boosted 800mg/d DRV (DRV800/r) and 1200mg/d (DRV1200/r), and boosted lopinavir 800mg/d (LPV/r) were evaluated. Plasma and urine were collected simultaneously. PI concentrations were measured with high performance liquid chromatography and spectrophotometric detection. Crystals were detected in fresh urine by polarized microscopy and identified according to their infrared spectrum.
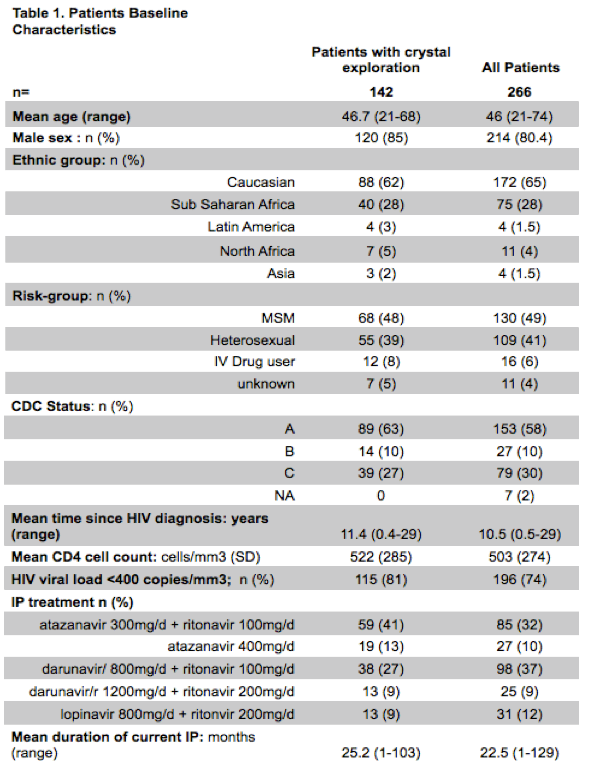
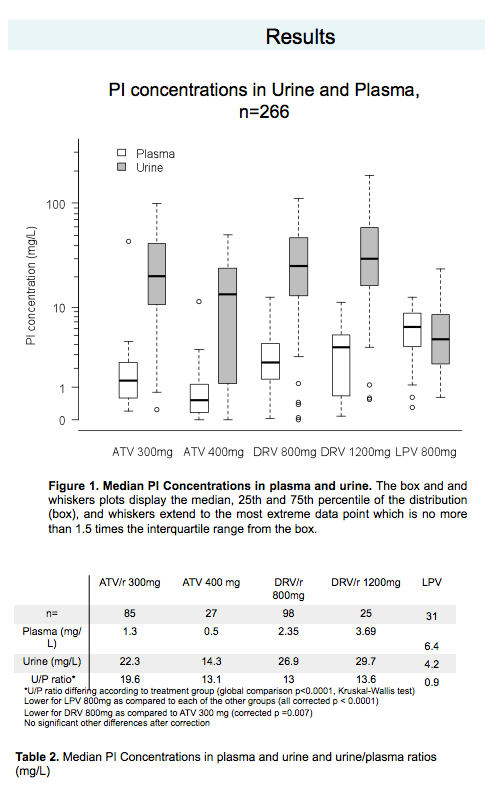
Results: 266 patients had PI dosage and crystals were searched in 149 of them. Mean age was 46 years [range 21-74]; 80% were male. Mean duration of HIV infection was 10 years [0.5-29] and current PI regimen had been stable for 22 months [3-129]. Mean CD4-cell counts was 503/mm3 [SD=274]; 74% had a HIV viral load < 400 copies/ml. Results from PI dosage are in Table 2. ATV, boosted or not, as well as DRV concentrate significantly more in urine than LPV (respectively, p<0.0001 and p<0.0001). ATV-crystals were found in the urine of 7 patients under ATZ (9%) and DRV-crystals in 4 patients receiving DRV (8%).
Conclusions: Unlike LPV, ATV and DRV reached high concentrations in urine and were associated with the detection of ATV crystals, in accordance with the risk of ATZ-induced renal toxicity but also DRV-crystals, which has never yet been described. Attention should be paid towards the potential renal toxicity of DRV.
INTRODUCTION
· ATV is one of the most prescribed protease inhibitor (PI) for first line antiretroviral therapy (ART) in HIV infected patients today.
· Rare cases of nephrolithiasis have been described, leading to obstructive uropathy and acute renal failure with ATV stones (1, 2, 3).
· Our objectives were to investigate possible risk-factors of urolithiasis in ATV-treated patients and understand why ATV and not other PI prescribed today lead to these complications.
ü Our hypothesis was that ATV may concentrate more in urine than other PI. We compared urinary and plasmatic PI concentrations in asymptomatic patients receiving different PI regimen.
ü Because urolithiasis is a rare event, we searched for the presence of PI-containing crystals in the urine of PI-treated patients, and explored potential risk-factors associated with the presence of PI-crystals.
Patients & Methods
HIV-infected adult patients receiving a stable ART regimen since at least 1 month were included if they received one of the following
· boosted 300mg/d ATV (ATV300/r)
· unboosted ATV 400mg/d (ATV400)
· boosted 800mg/d DRV (DRV800/r)
· boosted 1200mg/d DRV (DRV1200/r)
· boosted lopinavir (LPV800/r)
Blood and urine samples were collected simultaneously.
Both plasma and urinary PI levels were measured by a validated high-performance liquid chromatography with tandem mass spectrometry (HPLC/MS-MS), (range 0.05 to 10 mg/l - LOQ 0.01 mg/l).
Urine crystals were explored in patients with adequate urine samples only (ie. Sample received less than 2 hours after sampling, adequate sampling conditions). Crystals were detected by polarized microscopy on fresh urine all by the same investigator (MD) and infrared spectrophotometry was used to determine the composition of the crystals. Suspected PI crystals were compared to the spectrophotometry of a PI pill.
Statistics
Global comparison of the treatment groups were performed by Kruskal-Wallis test, post-hoc two-by-two group comparisons by Wilcoxon rank-sum test with p-values corrected for multiple testing using Holm's method.
Factors associated with the presence of crystals in urine were analyzed using Wilcoxon rank-sum tests and Fisher's exact tests. A p-value<0.05 was considered significant.
|
| |
|
 |
 |
|
|