 |
 |
 |
| |
Zinc Finger CD4 Increases: Preferential Expansion of Transitional and Central Memory CD4 T-cells following Adoptive Transfer of Zinc Finger Nuclease CCR5 Modified AutologousCD4 T-cells (SB-728-T)
|
| |
| |
Reported by Jules Levin
ICAAC Sept 2012 SF
SANGAMO BIOSCIENCES ANNOUNCES PRESENTATION OF CLINICAL DATA FROM ZFP THERAPEUTIC FOR HIV/AIDS AT ICAAC 2012 - slides from oral talk below
Findings Demonstrate Immune Reconstitution and Potential Success of "Functional Cure" in HIV-Infected Individuals
Richmond, California, September 10, 2012 - Sangamo BioSciences, Inc. (Nasdaq: SGMO) announced that data from its Phase 1 clinical programs to develop SB-728-T, a novel therapeutic approach designed to generate a "functional cure" for HIV/AIDS, were presented at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). The meeting is being held in San Francisco from September 9-12, 2012. "The immunologic data presented at ICAAC have predictive implications for the success of this exciting new therapeutic approach to HIV and the realization of a "functional cure' for the disease," commented Rafick-Pierre Sékaly, Ph.D., Co-Director & Chief Scientific Officer, the Vaccine & Gene Therapy Institute of Florida (VGTI Florida), whose laboratory carried out the analysis. "SB-728-T treatment results in an unprecedented and durable increase in CD4+ cells. Importantly, our analysis shows that this is primarily due to the expansion of CD4+ T-cell types that are vital for the successful reconstitution of the immune system in HIV-infected individuals - the central and transitional memory cells."
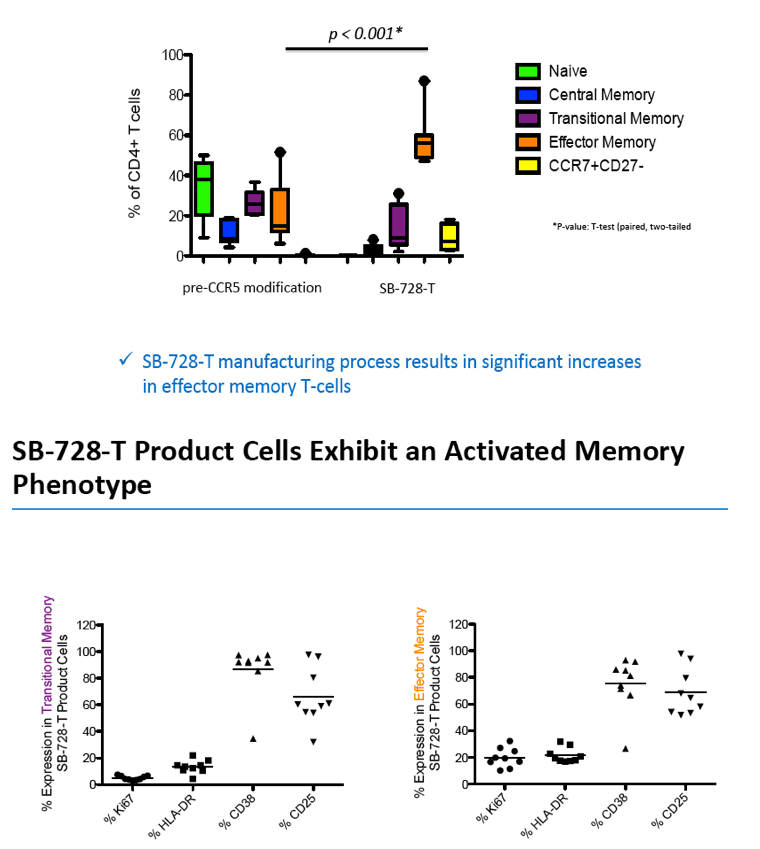
"These data are very important because CD4 T-cells, especially memory T-cells, are precisely the cell type that we would want to protect and expand to enable HIV-infected individuals to control infections, and HIV, without antiretroviral drugs," added Dale Ando, M.D., Sangamo's vice president of therapeutic development and chief medical officer. "Our aim is to provide a protected reservoir of immune memory cells to replenish the cells killed by HIV and to generate an effective immune response against the virus and opportunistic infections. Central and transitional memory T-cells remember previously encountered foreign invaders, such as viruses or bacteria. These cells can survive in the body for the individual's lifetime, and when they re-encounter the same antigen they reactivate, producing a faster and stronger immune response than the previous encounter. SB-728-T seems to both expand the total memory pool, and by CCR5 modification, protect a proportion of that pool from HIV entry, suggesting that SB-728 treatment has the potential to reconstitute and protect an effective and durable immune system in HIV-infected individuals." SB-728-T is generated by ZFN-mediated modification of the gene encoding the CCR5 receptor in a patient's own T-cells, disrupting the expression of this key co-receptor for HIV entry and rendering the modified cells resistant to HIV infection.
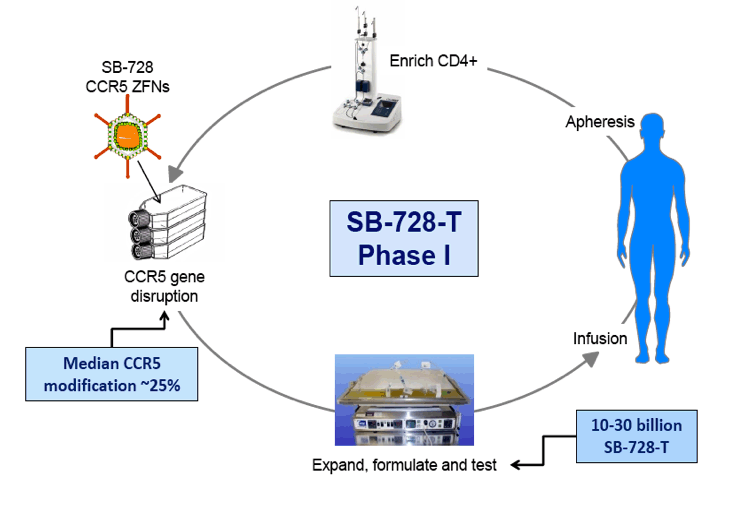
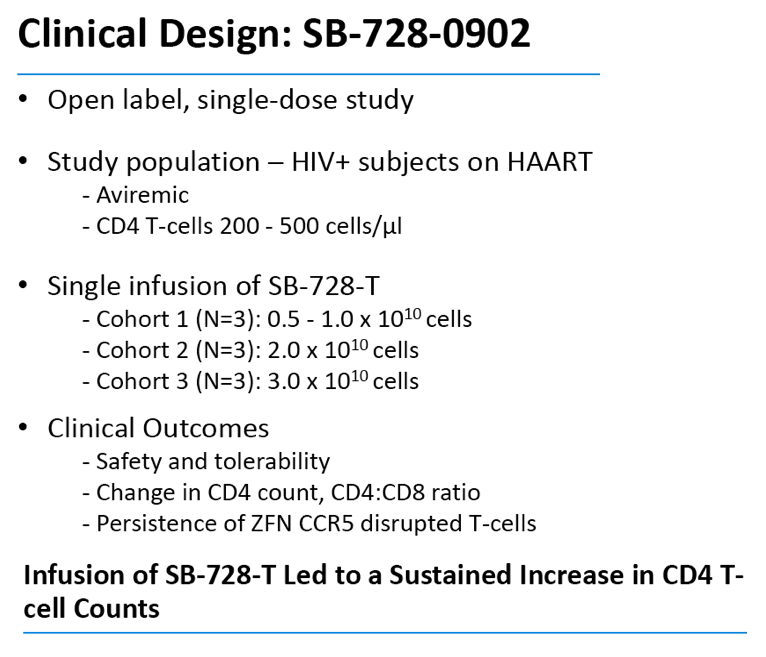
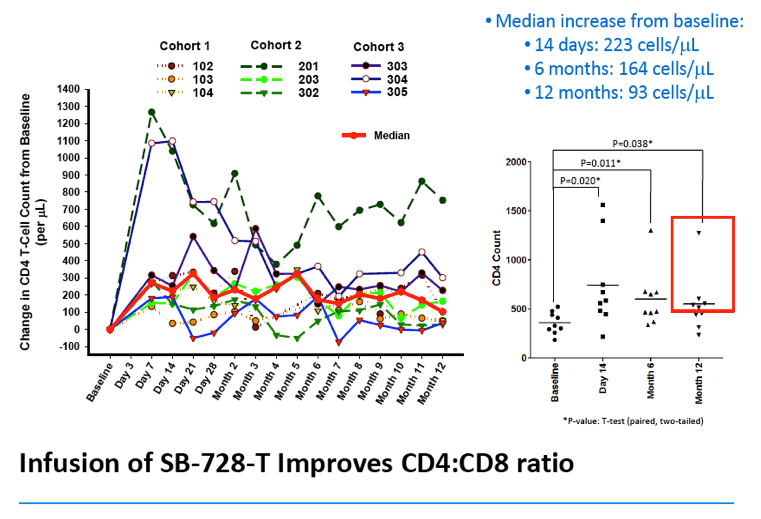
In an oral presentation made on Monday, September 10th, 2012 at ICAAC, data were presented from all dosing cohorts of Sangamo's Phase 1 dose-escalation study (SB-728-902) in subjects on highly active antiretroviral therapy (HAART). The data demonstrate that SB-728-T infusion in HIV-infected subjects is well-tolerated, and results in significant and sustained increases in CD4+ T-cells above baseline throughout the year-long period reported in the study. Statistically significant improvement in CD4 counts were observed even at 12 months post infusion (p<0.038). In particular, CD4 counts improved to greater than 500 cells/mm3 in five of nine subjects in the study at one year post-treatment, which is the usual T-cell count threshold for initiation of HAART in HIV-infected subjects.
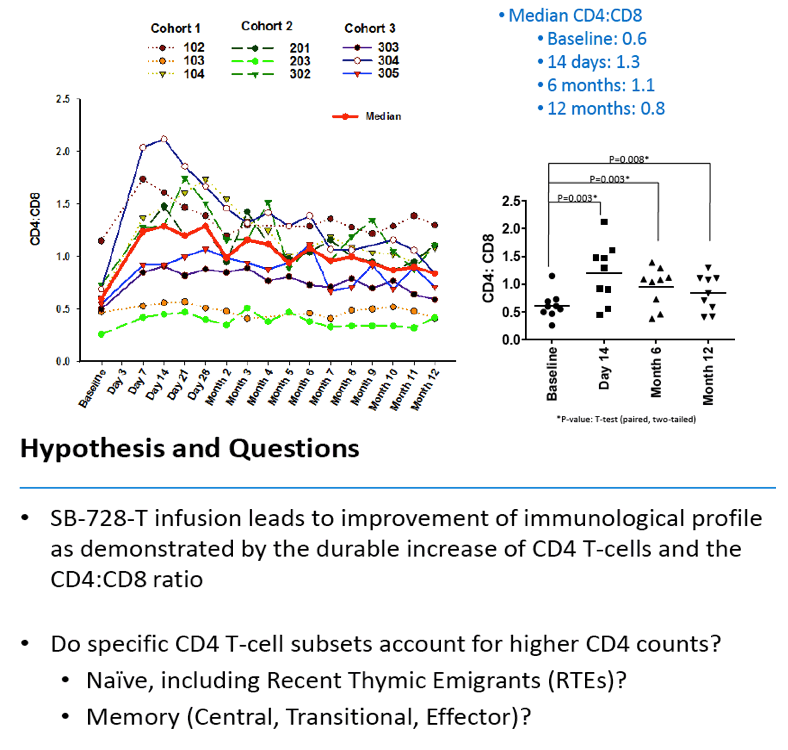
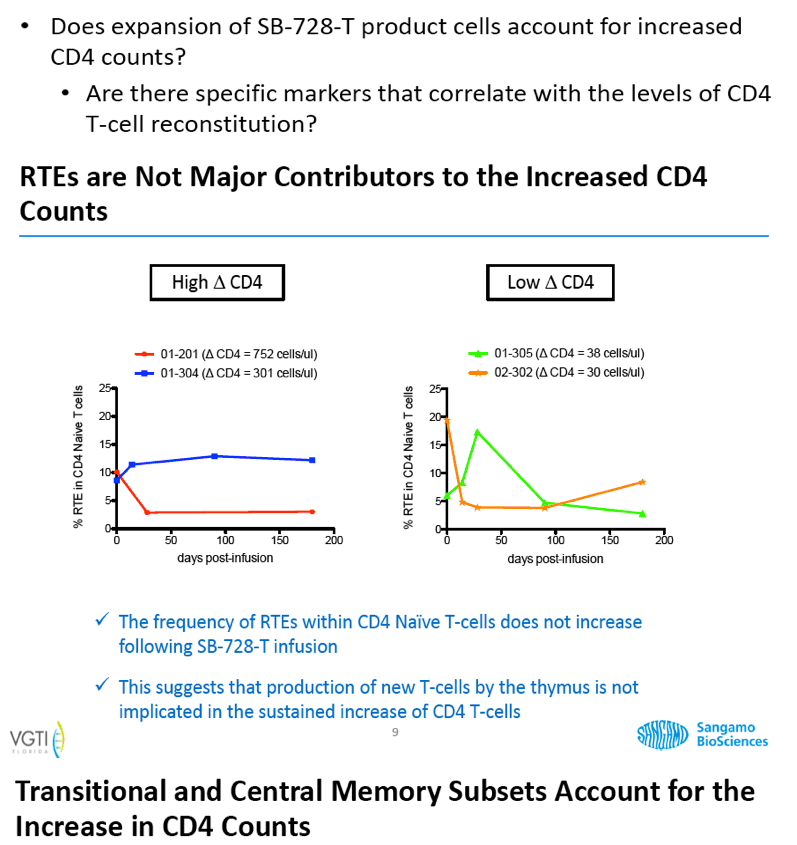
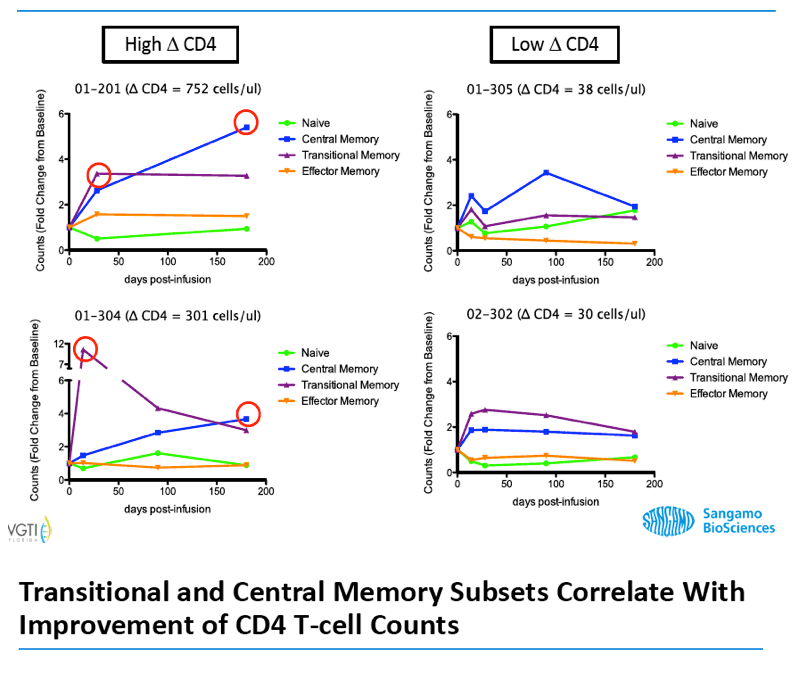
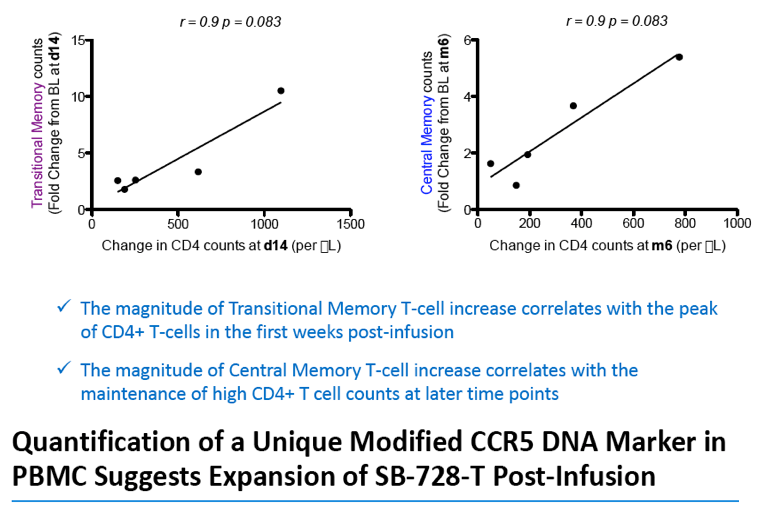
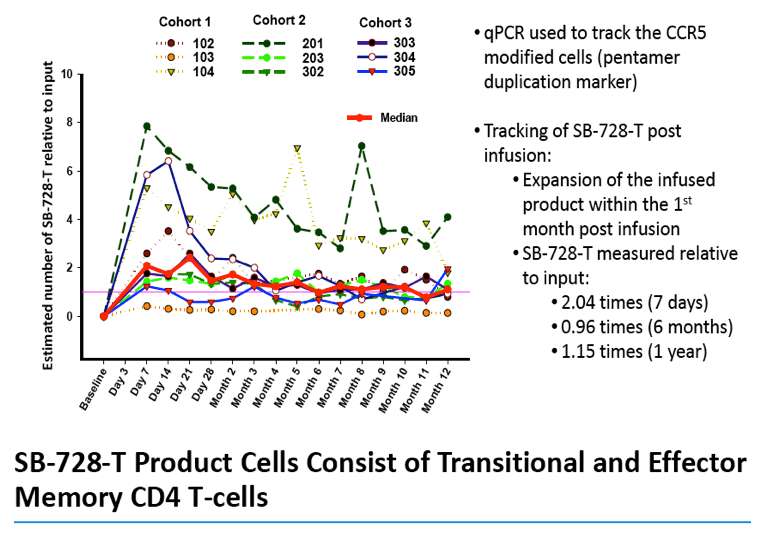
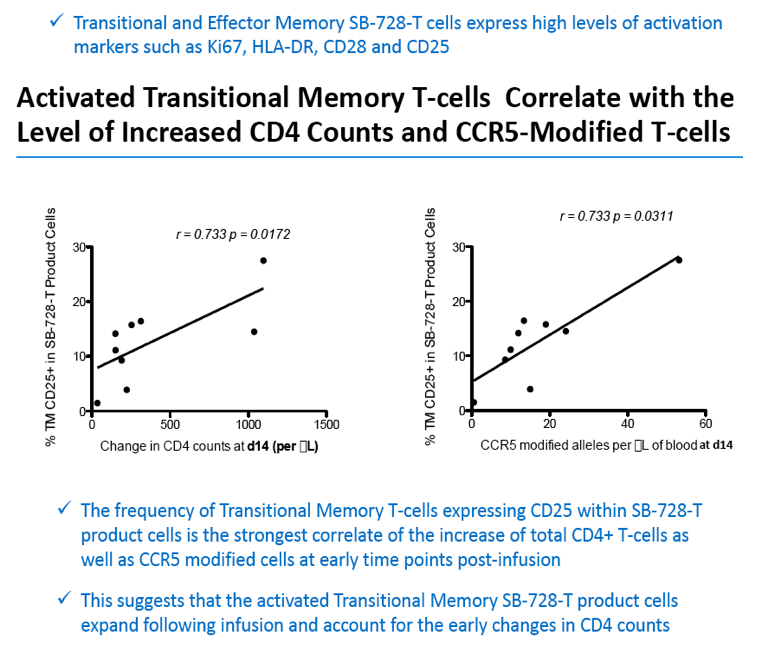
Analysis of the specific types of CD4 T-cells that comprise the initial increase in CD4+ T-cells post-infusion revealed that they were primarily transitional memory T-cells (TTM). The frequency of TTM expressing CD25 (a marker that identifies activated T-cells) within the SB-728-T product correlated with the peak CD4 count post-infusion (r=0.733, p=0.0172). This suggests that replication of activated TTM SB-728-T cells post-infusion accounts for the initial peak improvement in CD4+ T-cells. As the infused cells consist of only 1-10% of total memory cells at six months post-treatment, the prolonged increase in absolute numbers of CD4+ T-cells may be accounted for by the enhanced survival and differentiation of host central memory T-cells (TCM). Specifically, while the magnitude of the increase in TTM positively correlated with the peak of CD4+ T-cells in the first weeks post-infusion (r= 0.9, p=0.083), the increase in TCM correlated with the maintenance of high CD4+ T cell counts at later time points (r= 0.9, p=0.083). Proliferation of the SB-728-T product post-infusion was sustained over the year-long period reported in the study with median modified circulating cell numbers measured to be 2.04-fold relative to input at 7 days, 0.96-fold at 6 months and 1.15-fold at 1 year post-infusion.
These preliminary data confirm the prolonged engraftment of SB-728-T, and suggest that SB-728-T has the attributes to provide sustained improvement in the CD4 memory compartment and the potential to reconstitute the immune system in immune non-responders.
"Our Phase 1 trials continue to provide valuable insight into the durability and unprecedented effects of SB-728-T treatment on immune system health in HIV-infected individuals," said Geoff Nichol, M.B., Ch.B., Sangamo's executive vice president of research and development. "In addition we are making good progress in two Phase 2 clinical trials designed to maximize the engraftment of SB-728-T. We expect to present preliminary data in the first half of 2013 and a full data set in the second half of next year. The ground-breaking clinical data that we and our collaborators are generating continues to validate this treatment as a promising approach to provide a functional cure' for HIV/AIDS."
About SB-728-T
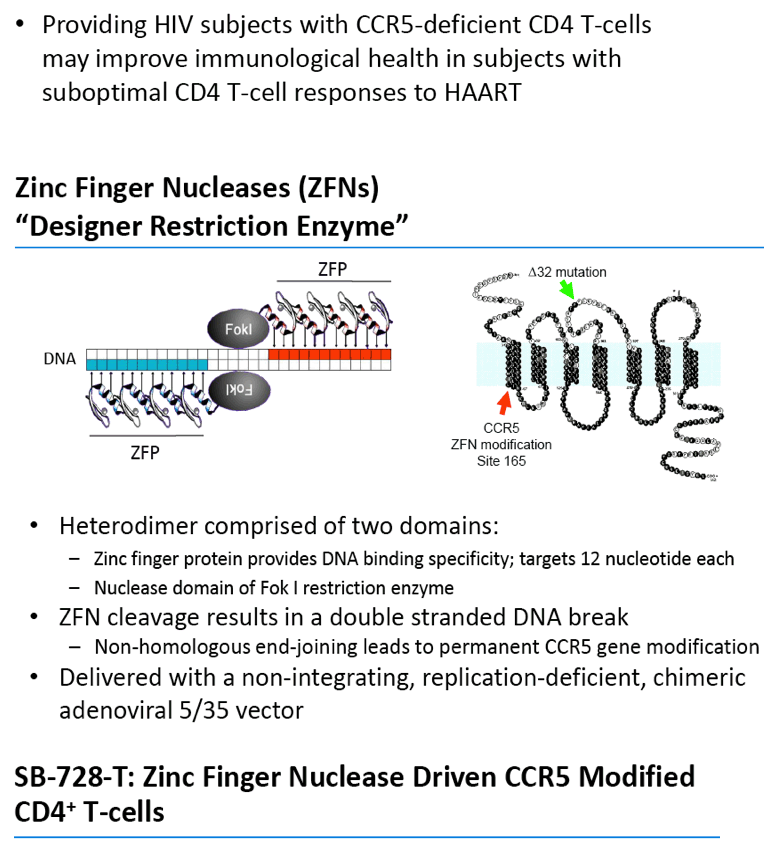
Sangamo's drug, SB-728-T, is generated by ZFN-mediated modification of the gene encoding the CCR5 receptor in a patient's own T-cells. ZFN modification disrupts the expression of this key co-receptor for HIV entry and renders cells resistant to HIV infection. The approach is based on the observation that a naturally occurring mutation in the CCR5 gene, CCR5 delta-32, provides protection from HIV infection. Individuals in whom both copies of the CCR5 gene carry the delta-32 mutation are generally not susceptible to the most common strain of HIV.
The non-employee authors of these abstracts have no financial relationship with Sangamo.
About Sangamo
Sangamo BioSciences, Inc. is focused on research and development of novel DNA-binding proteins for therapeutic gene regulation and genome editing. The Company has ongoing Phase 2 clinical trials to evaluate the safety and efficacy of a novel ZFP Therapeutic® for the treatment of HIV/AIDS. Sangamo's other therapeutic programs are focused on monogenic diseases, including hemophilia and hemoglobinopathies such as sickle cell anemia and beta-thalassemia, and a program in Huntington's disease. Sangamo's core competencies enable the engineering of a class of DNA-binding proteins known as zinc finger DNA-binding proteins (ZFPs). Engineering of ZFPs that recognize a specific DNA sequence enables the creation of sequence-specific ZFP Nucleases (ZFNs) for gene modification and ZFP transcription factors (ZFP TFs) that can control gene expression and, consequently, cell function. Sangamo has entered into a strategic collaboration with Shire AG to develop therapeutics for hemophilia, Huntington's disease and other monogenic diseases, and it has also established strategic partnerships with companies in non-therapeutic applications of its technology including Dow AgroSciences and Sigma-Aldrich Corporation. For more information about Sangamo, visit the company's website at www.sangamo.com.

|
| |
|
 |
 |
|
|