 |
 |
 |
| |
New Phase II Data from All-Oral Regimens
|
| |
| |
Reported by Jules Levin
AASLD 2013 Nov 1-4 Wash DC
Fred Poordad, MD
VP, Academic and Clinical Affairs
The Texas Liver Institute
Professor of Medicine
University of Texas Health Science Center
San Antonio, Texas
Dr Poordad noted this presentation did not include all the regimens in phase 2 & not all of them presented at this AASLD because of time constants of his time allotted for this talk.. there are additional regimens which I reported in the NATAP coverage, including these 2 noteworthy studies in African-Americans and HIV/HCV coinfected using IFN-free regimens:
AASLD: Combination Oral, Ribavirin Free, Antiviral Therapy to Optimize Treatment Outcomes for Hepatitis C Treatment Naïve Patients: Interim Results from the NIAID SYNERGY Trial (6 & 12 weeks therapy) - (11/06/13)
AASLD: All-Oral Therapy With Sofosbuvir Plus Ribavirin For the Treatment of HCV Genotype 1, 2, and 3 Infection in Patients Co-infected With HIV (PHOTON-1) - (11/06/13)
64rd Annual Meeting of the American Association for the Study of Liver Diseases
Washington, DC Nov 1-5 2013
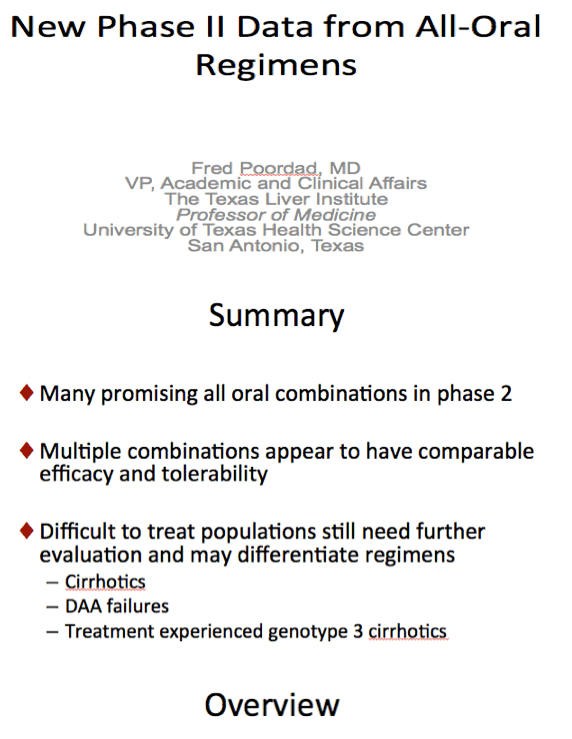
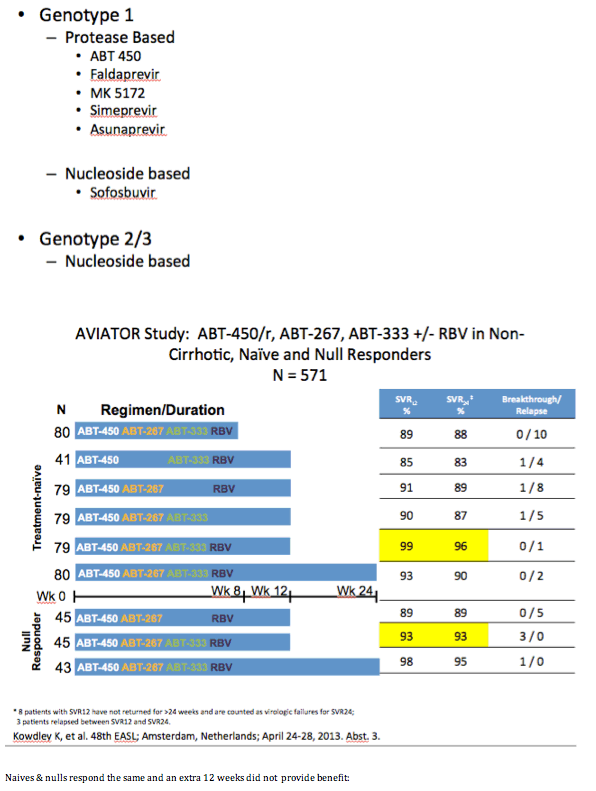
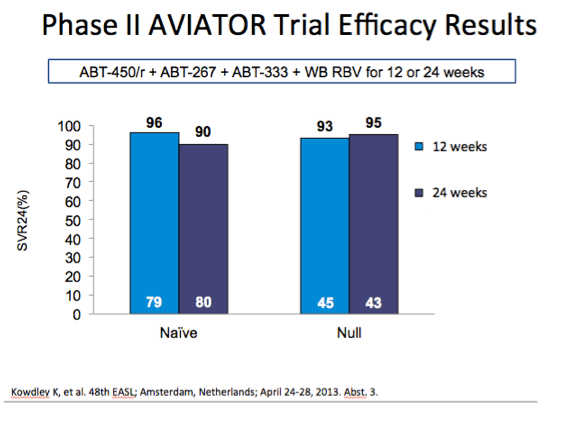
from Jules: As Dr Poordad made reference to - Previous negative predictive factors did not affect SVR rates here. Even in this null
responder population responses were the same: male vs female, 1a vs 1b, high baseline viral load vs low baseline viral load, F0-1
vs F2-3 but this analysis did not include cirrhotics (we will look to phase 3 for that), and CC vs non-CC.
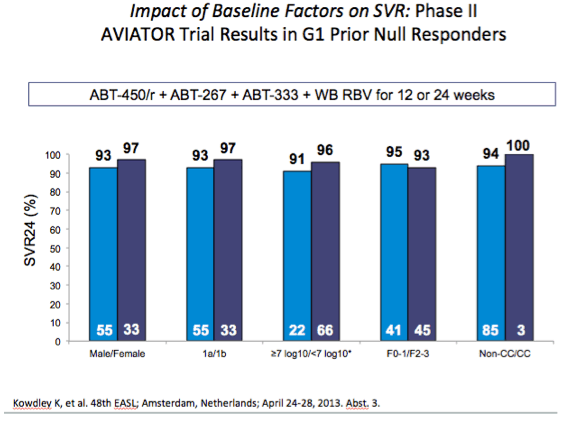
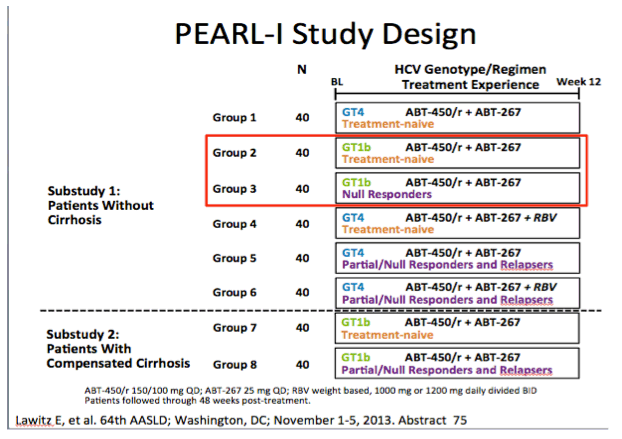
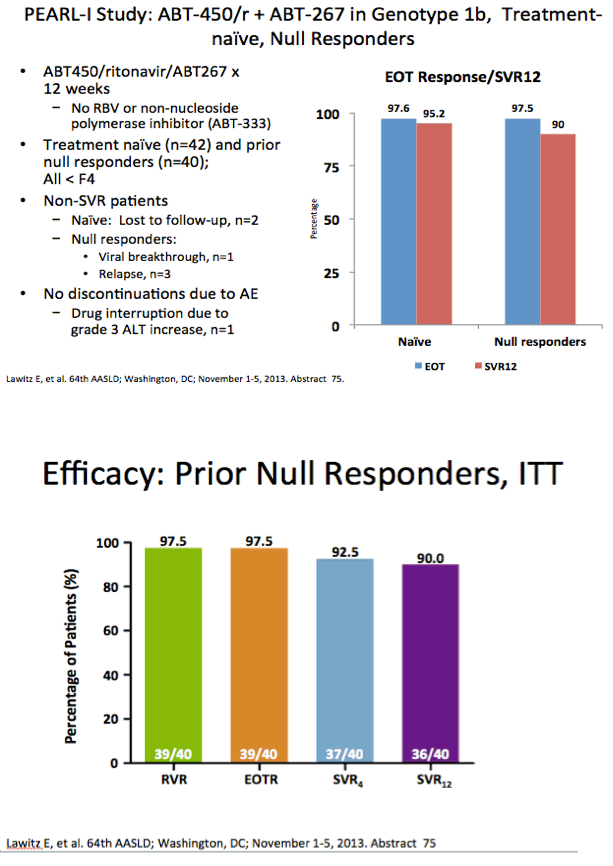
AASLD: Interferon- and Ribavirin-free Regimen of ABT-450/r + ABT-267 in HCV Genotype 1b-infected Treatment-naïve Patients and Prior Null Responders - (11/04/13)
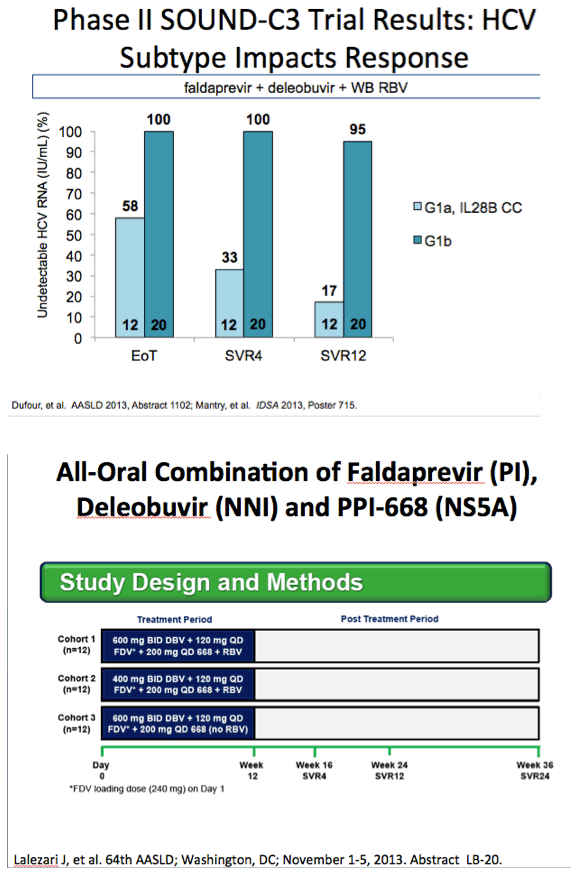
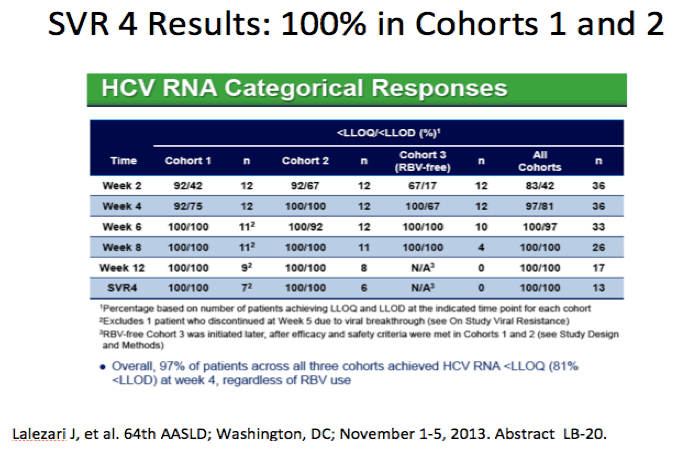
AASLD: High Efficacy and Safety of the All-Oral Combination Regimen, MK-5172 / MK-8742 ± RBV for 12 Weeks in HCV Genotype 1 Infected Patients: The C-WORTHY Study - (11/05/13)
AASLD: Efficacy and Safety of an Interferon-Free Regimen of MK-5172 + Ribavirin for 12 Weeks or 24 Weeks in Treatment-Naive, Noncirrhotic Subjects With HCV GT1 Infection: The C-SPIRIT Study - (11/04/13)
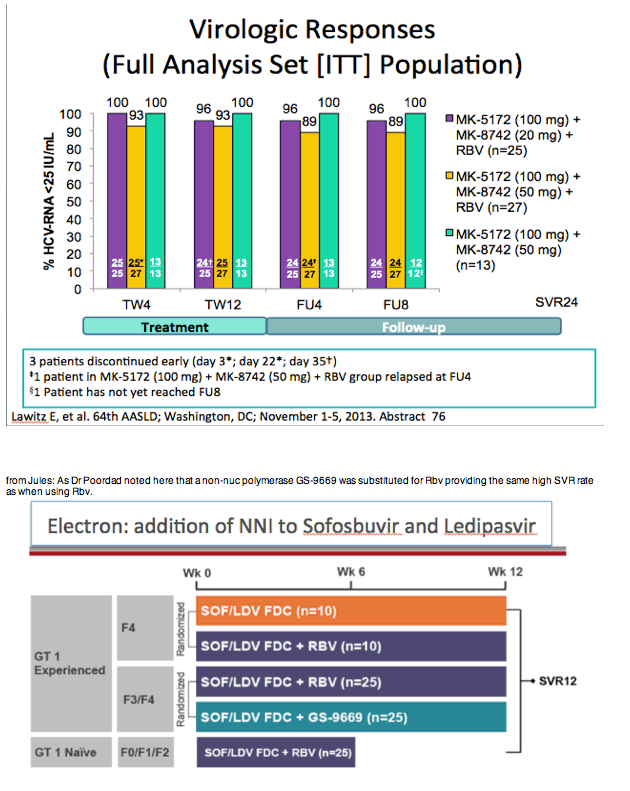
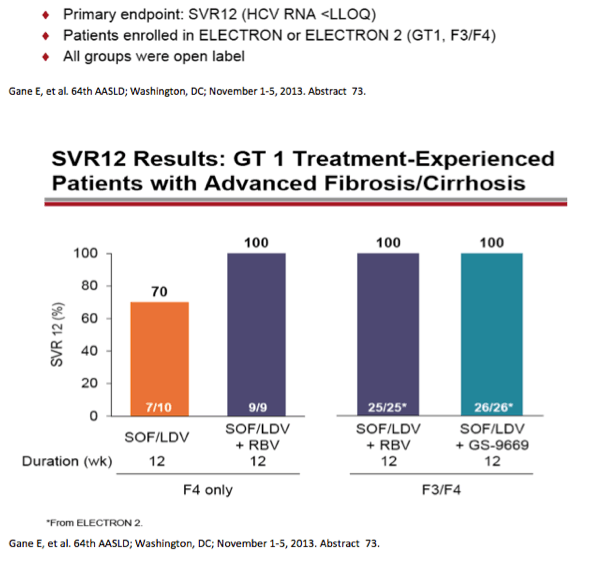
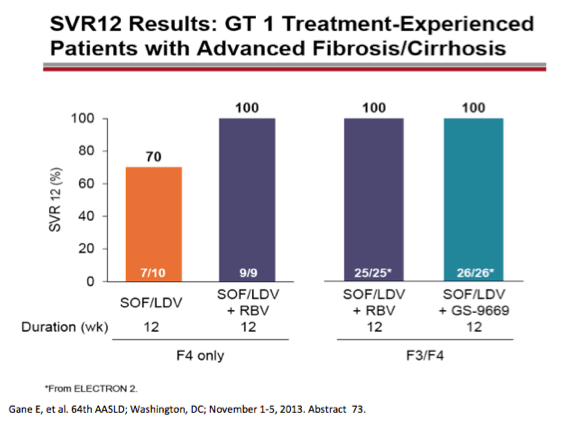
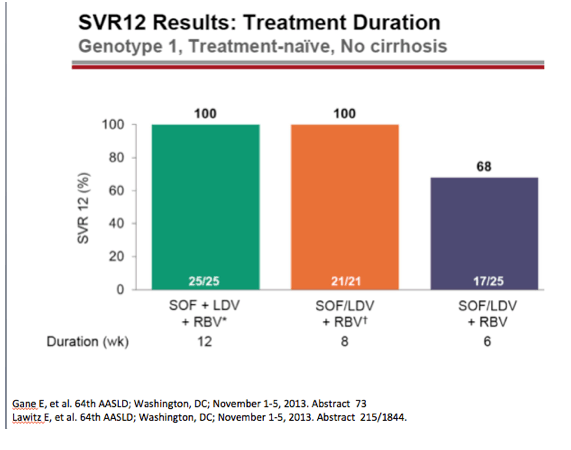
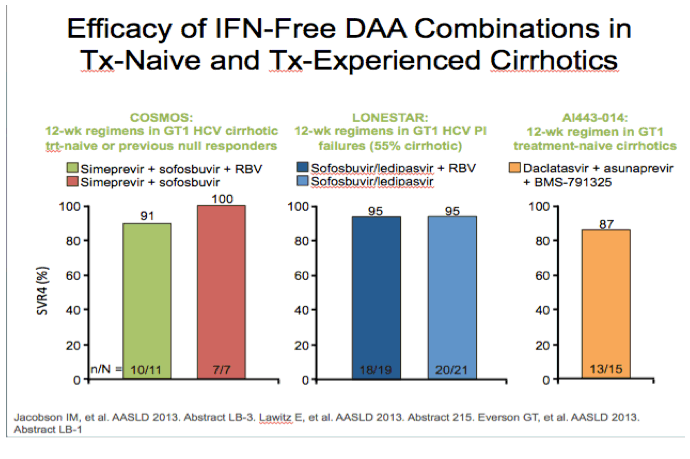
from jules: As noted by Dr Poordad in the LONESTAR study addressing duration of therapy, the number of patients is small but
8 weeks and 12 weeks with SOFosbuvir+Ledipasvir+Rbv in treat-naive non-cirrhotics yielded the same SVR rate but 6 weeks with
this regimen was not as good. In the LONESTAR Study which you can see by clicking the link just below HCV protease inhibitor
failures recd the same regimen for 12 weeks with & without Rbv & 50% were cirrhotic & this yielded 21/21 achieving SVR12 with
Rbv & 18/19 without Rbv achieved SVR12.
AASLD: Sofosbuvir and Ledipasvir Fixed-Dose Combination with and without Ribavirin in Treatment-Naïve and Previously Treated Patients with Genotype 1 Hepatitis C: The LONESTAR Study - (11/06/13)
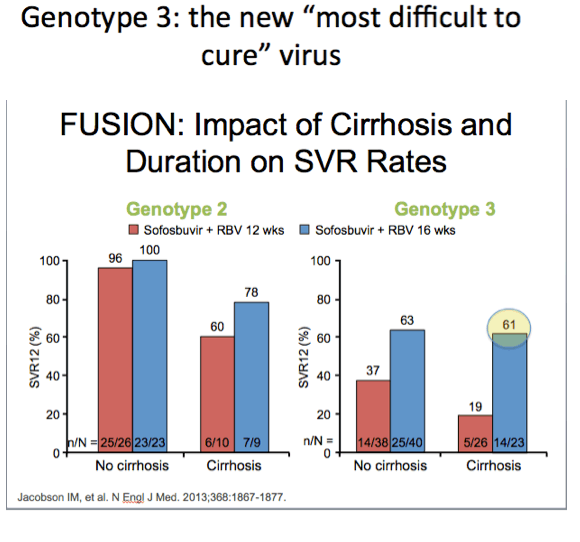
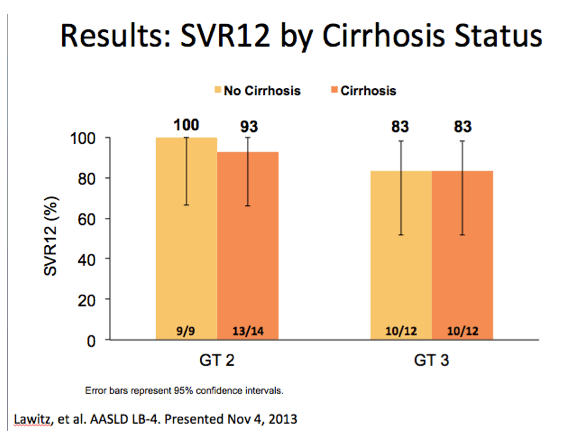
Dr Poordad commented regarding the far left bar that the cirrhotic treat-exp GT3 still had only 60% SVR rates suggesting that perhaps prior
experience with Peg/Rbv may have for some reason disadvantaged this patient group. (from Jules: this has been a theme mentioned
by several researchers at several recent conferences which of course is interesting & perhaps true but I don't think there i
s any evidence of this theory).
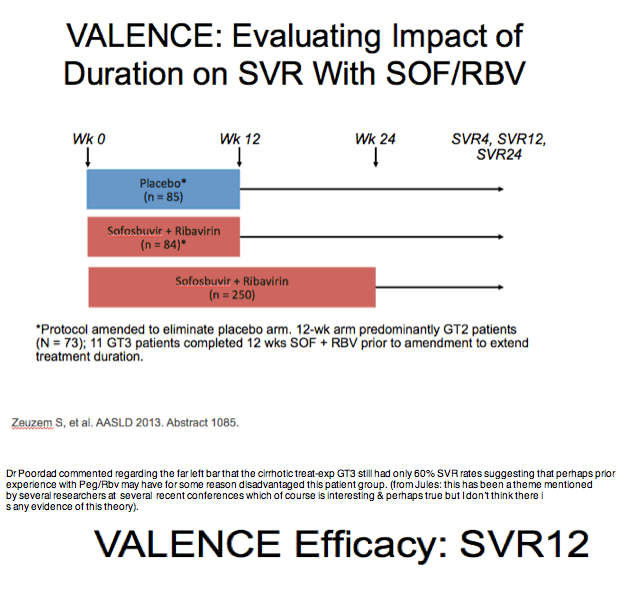
BUT IN THIS STUDY the same group of Treatment-Exp recd 12 weeks of Peg/rbv + Sofosbuvir with a 83% SVR rate, Dr Poordad
said this suggests this might be the best treatment for GT3 treat-exp cirrhotics.
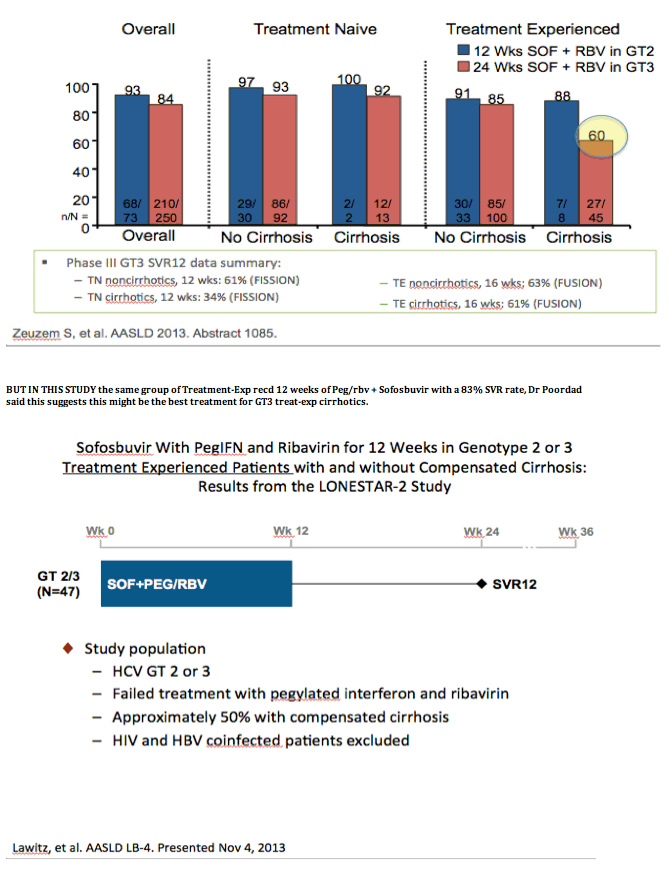
from Jules: 2 relapsers & 2 lost to discontinuations in the GT3 group, I think 1 relapser & 1 discontinuation in the 10/12 in the GT3 cirrhotic group,
and the same in the non-cirrhotic group.
AASLD: Sofosbuvir in Combination With PegIFN and Ribavirin for 12 Weeks Provides High SVR Rates in HCV-Infected Genotype 2 or 3 Treatment- Experienced Patients with and without Compensated Cirrhosis: Results from the LONESTAR-2 Study - (11/05/13)
|
| |
|
 |
 |
|
|