 |
 |
 |
| |
Gilead at AASLD: Sofosbuvir for GT 3, 2 and 1
|
| |
| |
from Jules: below are links to the LONESTAR2 & VALENCE studies presented at AASLD Nov 1-5 2013 in Wash DC which reported results in GT2 & 3 patients with IFN-free Sofosbuvir+Rbv or Sofosbuvir+Peg/Rbv, and in LONESTAR2 83% of treatment-experienced patients with GT3 achieved SVR after 12 weeks therapy, both cirrhotics & non-cirrhotics. For the harder to treat cirrhotic treatment experienced in the VALENCE Study who recd Sofosbuvir+Rbv for 24 weeks the SVR was 60% but treatment-naives & non cirrhotic treatment experienced did much better with 87-94 SVR rates. In large phase 3 studies now is Sofosbuvir+Ledipasvir (nucleotide+NS5A inhibitor) which in LONESTAR, link below, achieved high SVR rates in GT1 with 12 weeks therapy. Abbvie is in large phase 3 now too. Both Gilead & Abbvie are expected to submit their applications to the FDA for approval in the first half of 2014, no later than June 2014. In the meantime, phase 2 study of IFN-free Sofosbuvir+Simeprevir results from the COSMOS are linked to below with high SVR rates, and phase 2 study of Daclatasvir+Sofosbuvir achieved 100% SVR rates in prior HCV protease failures & in treatment-naives. In phase 2 are other IFN-free regimens with similarly high SVR rates in GT1 by Boehringer Ingelheim/Presidio, Merck, & BMS who reported their data at this AASLD. Vertex has a nucleotide, same class of drug as Sofosbuvir in phase 2 being studied in IFN-free combinations with various other HCV drugs (DAAs) and Achillion presented at AASLD a poster of their nucleotide in preclinical, and Idenix announced they are entering clinical trial with their new nucleotide.
64rd Annual Meeting of the American Association for the Study of Liver Diseases
Washington, DC Nov 1-5 2013
AASLD: Sofosbuvir and Ledipasvir Fixed-Dose Combination with and without Ribavirin in Treatment-Naïve and Previously Treated Patients with Genotype 1 Hepatitis C: The LONESTAR Study - (11/06/13)
AASLD: Once Daily Sofosbuvir/Ledipasvir Fixed-Dose Combination With or Without Ribavirin: Data From the ELECTRON Trials - (11/04/13)
GT1 African-Americans - AASLD: Combination Oral, Ribavirin Free, Antiviral Therapy to Optimize Treatment Outcomes for Hepatitis C Treatment Naïve Patients: Interim Results from the NIAID SYNERGY Trial (6 & 12 weeks therapy) - (11/06/13)
AASLD: All-Oral Therapy With Sofosbuvir Plus Ribavirin For the Treatment of HCV Genotype 1, 2, and 3 Infection in Patients Co-infected With HIV (PHOTON-1) - (11/06/13)
AASLD: GILEAD ANNOUNCES PHASE 3 RESULTS FOR AN ALL-ORAL, SOFOSBUVIR-BASED REGIMEN FOR THE TREATMENT OF HEPATITIS C IN PATIENTS CO-INFECTED WITH HIV - (11/02/13)
AASLD: SVR results of a once-daily regimen of simeprevir (SMV, TMC435) plus sofosbuvir (SOF, GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naïve and prior null responder patients: The COSMOS study - (11/05/13)
AASLD: Sofosbuvir Plus Ribavirin in the Treatment of Chronic HCV Genotype 4 Infection in Patients of Egyptian Ancestry - (11/11/13)
AASLD: Lack of a Clinically Important Pharmacokinetic Interaction Between Norgestimate/Ethinyl Estradiol and Sofosbuvir (SOF) or Ledipasvir (LDV) in HCV-Uninfected Female Subjects - (11/11/13)
AASLD: Pharmacokinetics of Ledipasvir, an HCV-Specific NS5A Inhibitor, in HCV-Uninfected Subjects With Moderate or Severe Hepatic Impairment - (11/11/13)
AASLD: No Resistance Detected in Four Phase 3 Clinical Studies in HCV Genotypes 1-6 of Sofosbuvir + Ribavirin With or Without Peginterferon - (11/07/13)
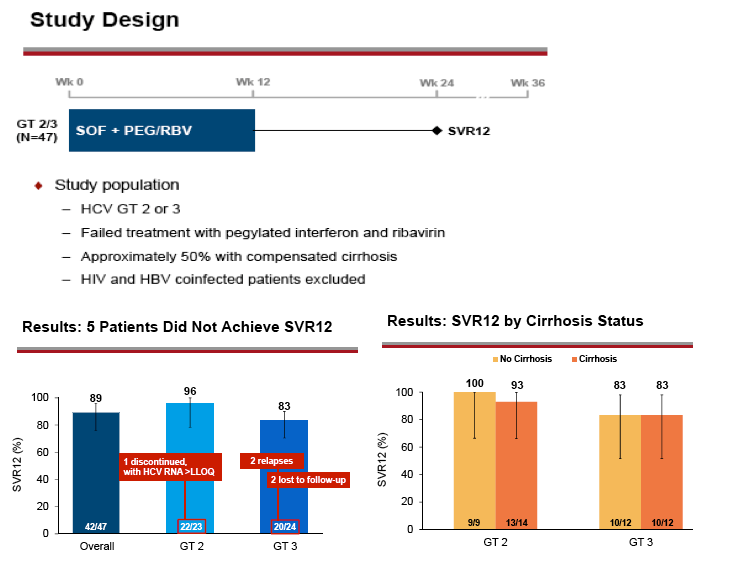
AASLD: Sofosbuvir in Combination With PegIFN and Ribavirin for 12 Weeks Provides High SVR Rates in HCV-Infected Genotype 2 or 3 Treatment- Experienced Patients with and without Compensated Cirrhosis: Results from the LONESTAR-2 Study - (11/05/13)
The LONESTAR-2 study aimed to:
- Evaluate the efficacy of a 12-week SOF + PEG/RBV regimen in treatment-experienced patients with GT 2 or 3 infection, including those with cirrhosis
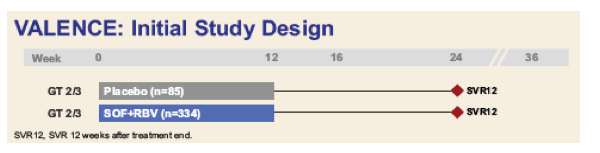
AASLD: Sofosbuvir + Ribavirin for 12 or 24 Weeks for Patients With HCV Genotype 2 or 3: the VALENCE Trial - (11/04/13)
VALENCE (NCT01682720) is a Phase 3 study conducted in Europe assessing safety and efficacy of SOF+RBV administered for:
- 12 weeks in patients with HCV GT 2
- 24 weeks in patients with HCV GT 3
Treatment-naïve or -experienced patients with HCV GT 2 or 3 were originally randomized 4:1 to receive SOF+RBV for 12 weeks or matching placebo
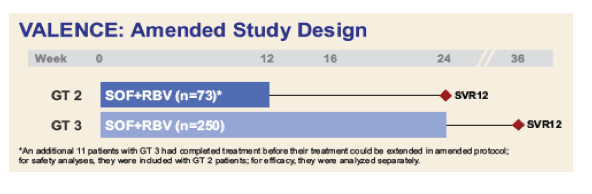
Due to emerging data suggesting GT 3 patients would benefit from >12 weeks of treatment, the study was subsequently amended
- Treatment was extended to 24 weeks for GT 3 patients, irrespective of treatment history
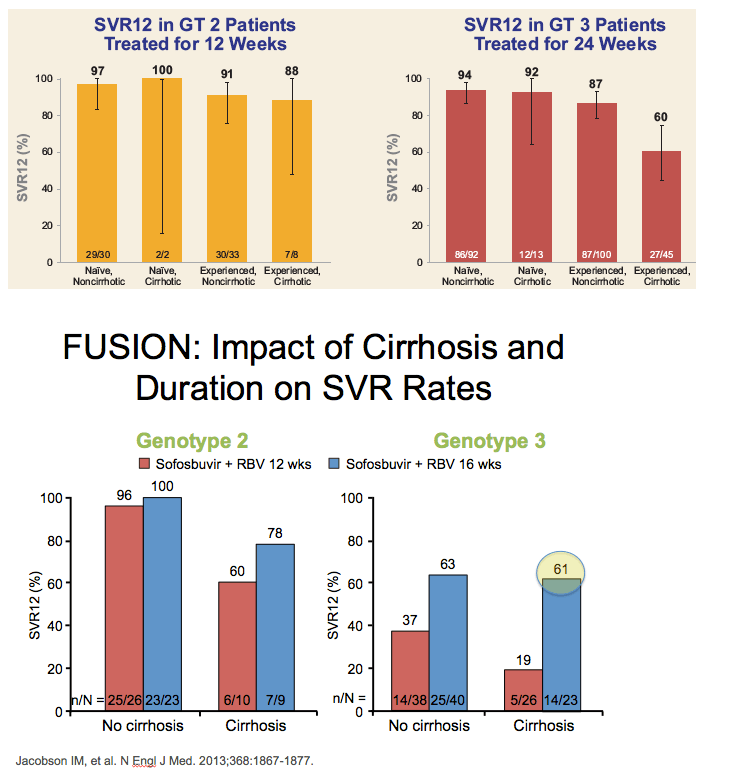
CONCLUSIONS:
- SOF + RBV for 24 weeks demonstrated SVR rates >90% in treatment-naïve HCV GT 3 patients
- In treatment-experienced HCV GT 3 patients, SVR rates were 60% and 87% in those with and without cirrhosis, respectively
- GT 2 patients had high SVR rates following treatment with SOF+RBV for 12 weeks
- No resistance detected in any patient with relapse SOF+RBV for 12 or 24 weeks was well tolerated
- Safety profile consistent with that of RBV
- No additional adverse events when treatment was extended to 24 weeks
AASLD: Sofosbuvir Selects the NS5B S282T Mutation In Vitro in Genotype 1-6 Replicons and Is Not Cross-Resistant to Resistance-Associated Variants Selected by Other Classes of Antiviral Inhibitors - (11/14/13)
AASLD: Gilead Announces Phase 2 Results for Sofosbuvir-Based Regimens in Hepatitis C Patients Before and After Liver Transplantation - (11/02/13)
AASLD: Sofosbuvir and Ribavirin for the Treatment of Established Recurrent Hepatitis C Infection After Liver Transplantation: Preliminary Results of a Prospective, Multicenter Study - (11/05/13)
AASLD: Initial Evaluation of the Sofosbuvir Compassionate Use Program for Patients With Severe Recurrent HCV Following Liver Transplantation - (11/06/13)
AASLD: Pretransplant Sofosbuvir and Ribavirin to Prevent Recurrence of HCV Infection After Liver Transplantation - (11/06/13)
AASLD: Deep Sequencing of HCV NS5A From a 3-Day Study of GS-5816 Monotherapy Confirms the Potency of GS-5816 Against Pre-Existing Genotype 1-3 NS5A Resistance-Associated Variants - (11/05/13)
AASLD: GS-5816, a Once-Daily NS5A Inhibitor, Demonstrates Potent Antiviral Activity in Patients With Genotype 1-4 HCV Infection in a 3 Day Monotherapy Study - (11/04/13)
AASLD: Lack of a Clinically Significant Pharmacokinetic Drug-Drug Interaction Between Sofosbuvir and GS-5816 in Healthy Volunteers - (11/14/13)
AASLD: On-Treatment HCV RNA as a Predictor of Virologic Response in Sofosbuvir-Containing Regimens for Genotype 2/3 HCV Infection: Analysis of the FISSION, POSITRON, and FUSION Studies - (11/14/13)
AASLD: Targeting lysyl oxidase like 2 (LOXL2) inhibits collagen cross-linking and accelerates reversal of pre-established liver fibrosis - (11/14/13)
AASLD: Nucleotide Analog Levels in Liver Explants From HCV Infected Subjects Undergoing Liver Transplantation After Up to 24 Weeks Sofosbuvir (GS-7977) With Ribavirin Treatment - (11/14/13)
AASLD: Population Pharmacokinetics of Sofosbuvir and Its Major Metabolite (GS-331007) in Healthy and HCV-Infected Adult Subjects - (11/14/13)
AASLD: Efficacy and Safety of Sofosbuvir in Patients According to Fibrosis Stage: An Analysis of Phase 3 Data - (11/11/13)
|
| |
|
 |
 |
|
|