 |
 |
 |
| |
48 weeks outcomes of atazanavir/ritonavir monotherapy as maintenance strategy in HIV-1 treated subjects with viral suppression: interim analysis results of the MODAt Study
|
| |
| |
Reported by Jules Levin
EACS 2013 - 15th European AIDS Conference, Oct 16-19, Brussels, Belgium
A. Castagna1, V. Spagnuolo1,2, L. Galli1, C. Vinci1, S. Nozza1, E. Carini1,
A. d'Arminio Monforte3, F. Montella4, A. Antinori5, A. Di Biagio6, S.Rusconi7, A. Lazzarin1,2
1 Department of Infectious Diseases, IRCCS San Raffaele, Milan, Italy 2. UniversitÓ Vita-Salute San Raffaele, Milan, Italy
3. Department of Health Sciences, S Paolo Hospital, University of Milan, Milan, Italy
4. Division of Infectious Diseases Azienda Ospedaliera San Giovanni Addolorata, Rome, Italy
5. Clinical Department, National Institute for Infectious Diseases IRCCS Lazzaro Spallanzani, Rome, Italy
6. Clinic of Infectious Diseases, IRCCS AOU San Martino-IST, Genoa Italy.
7. Division of Infectious Diseases, Ospedale Luigi Sacco, University of Milan, Italy
PROGRAM ABSTRACT
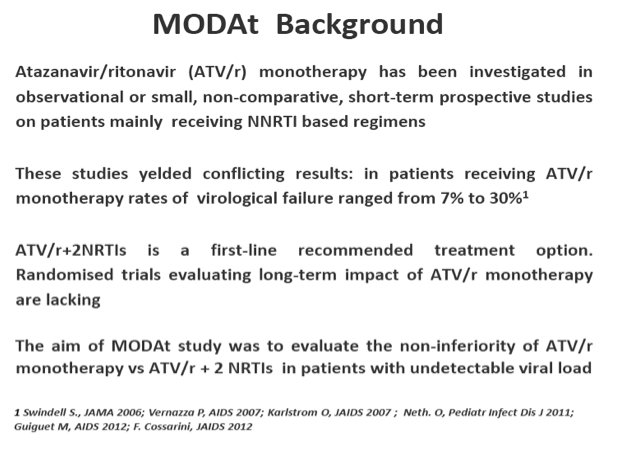
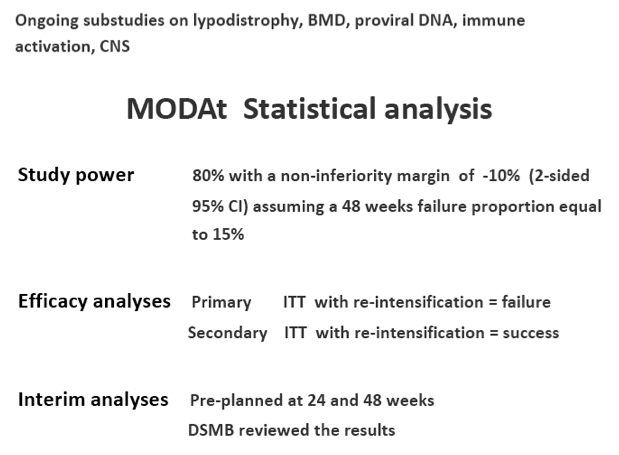
Objectives: To assess the non-inferiority of 48-weeks efficacy of a monotherapy strategy with atazanavir/ritonavir (ATV/r) vs atazanavir/ritonavir+2 NRTIs, in patients(pts) with no previous virologic failure (VF).
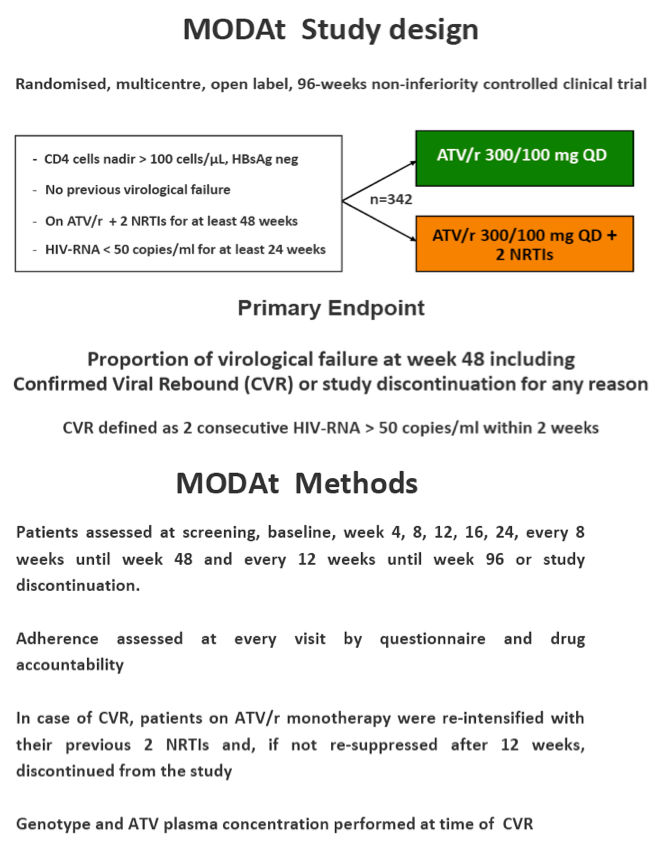
Methods: Multicentric, randomized, open-label, non-inferiority trial (80% power with delta =-10%). Pts on ATV/r 300/100mg+2 NRTIs since ≥48 weeks, suppressed since ≥24 weeks, HBsAg-negative, no previous VF were randomized to receive ATV/r-monotherapy or to maintain ATV/r triple-therapy.
Treatment failure (TF) was defined as confirmed VF (2 consecutive HIV-RNA>50 cp/ml; CVF) or discontinuation for any cause. In the ATV/r arm, patients with CVF re-introduced their previous NRTIs and remained in the study if HIV-RNA< 50 copies/mL within 12 weeks since re-intensification.
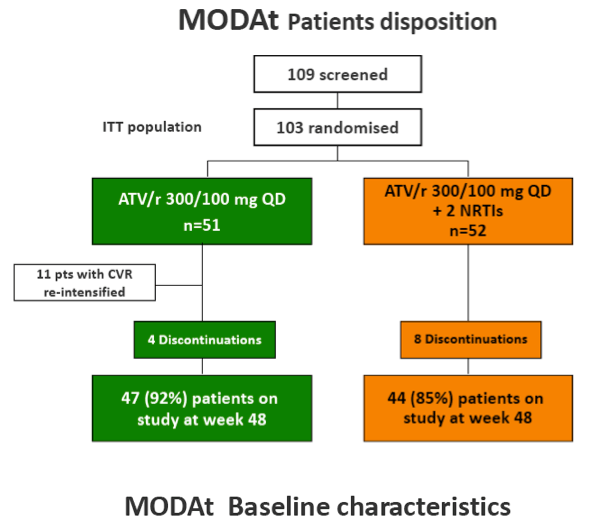
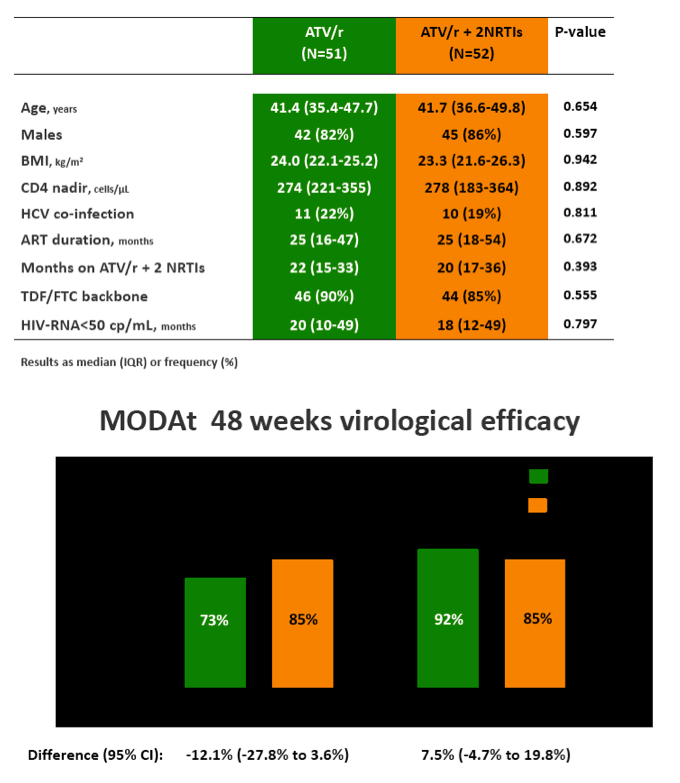
Results: 103 patients, 84% males, 13% HCV-infected, median(IQR) age of 42(36-48) years and baseline CD4+ 575(432-744) cells/ÁL.
In the 48-weeks interim analysis (ITT with TF=failure), efficacy was 73% (37/51 patients) in the monotherapy arm and 85% (44/52) in the triple-therapy arm (difference: -12.1%, 95% CI: -27.8 to 3.6); DSMB recommended to stop the study enrolment.
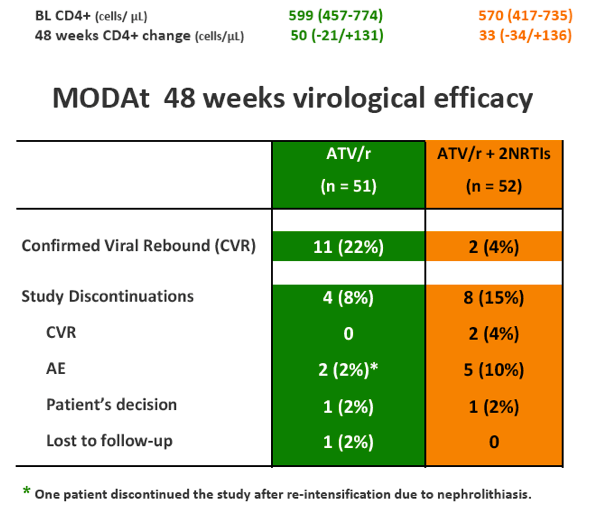
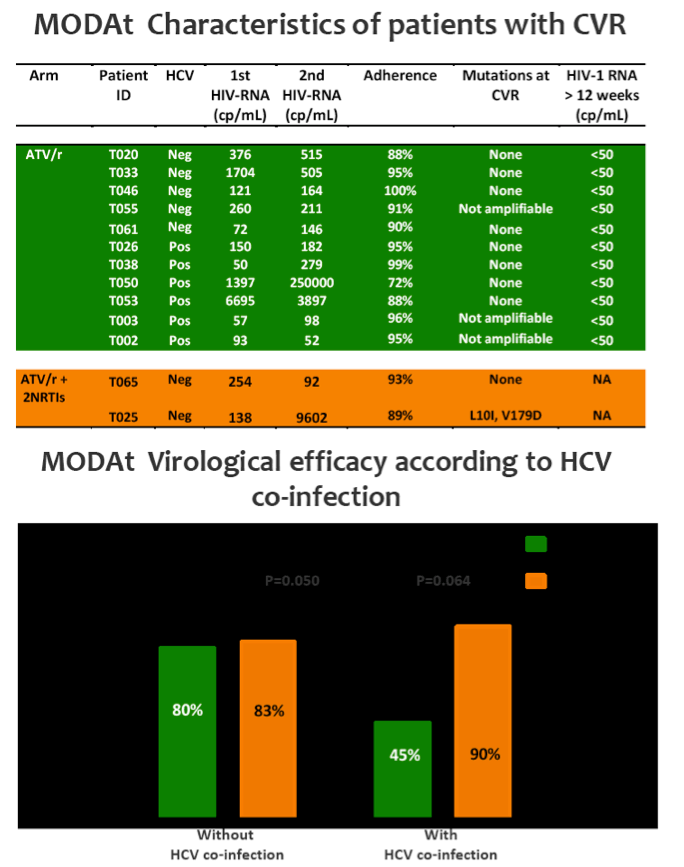
Eleven pts in monotherapy and 2 in triple-therapy had CVF. At CVF no mutations for PIs or NRTIs were observed and median HIV-RNA was 197(134-510) cp/ml; CVFs were more frequent in HCV-coinfected pts (56% vs 17%; p=0.024). All re-intensified pts re-suppressed viraemia after re-intensification. By a re-intensification-included analysis, 47(92%) in monotherapy and 44(85%) subjects in triple-therapy had HIV-RNA< 50 copies/mL at week 48(p=0.358).
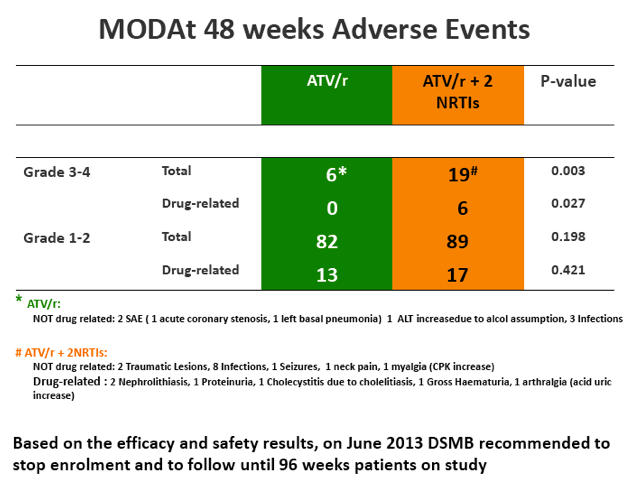
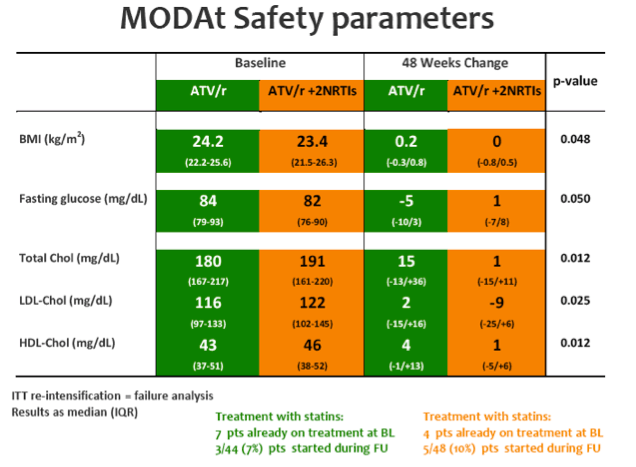
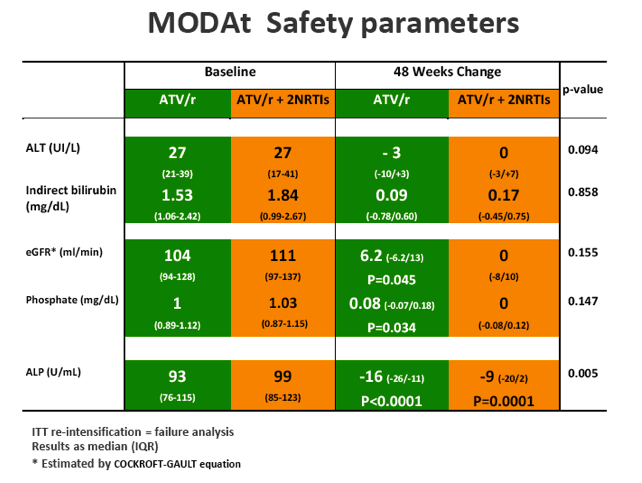
SAEs occurred in 2(4%) of 51 patients in the monotherapy arm. Overall grade 3-4 adverse events(AEs) were less frequent in monotherapy arm compared with triple-therapy [4 vs 19; p=0.0007]. A detailed description of AEs in Table 1
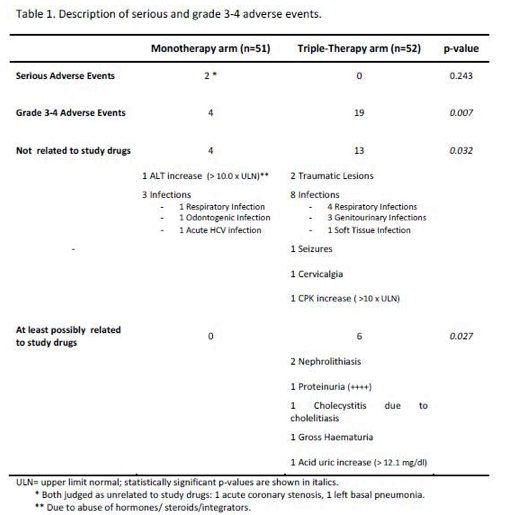
[Table 1]
Conclusions: In this interim analysis, 48-weeks CVF was more frequent in ATV/r monotherapy as compared to ATV/r triple-therapy. No mutation were detected at CVF and re-intensification was successful in all pts. Safety profile was more favourable in monotherapy arm.

|
| |
|
 |
 |
|
|