 |
 |
 |
| |
Bone Turnover Marker Changes Early in ART Predict Bone Loss After 96 Weeks
|
| |
| |
14th European AIDS Conference. October 16-19, 2013. Brussels.
Mark Mascolini
Changes in markers of bone resorption (destruction) and formation early in the course of antiretroviral therapy (ART) predicted clinically significantly bone loss after 96 weeks of treatment in a trial that randomized people to either raltegravir or tenofovir/emtricitabine (TDF/FTC), both with lopinavir/ritonavir [1]. Adding to the complexity of bone marker studies, results of this analysis indicated that greater increases in bone formation markers through 16 weeks of treatment were associated with clinically meaningful BMD loss at week 96.
Previous research has reported that bone mineral density (BMD) drops 2% to 6% in the 2 years after antiretroviral therapy begins regardless of the antiretrovirals taken [2]. When treatment starts, bone resorption markers increase faster than bone formation markers, creating what these researchers call a "catabolic window." Because it remains unclear whether early changes in turnover markers predict antiretroviral-associated bone loss, these investigators addressed that question in PROGRESS study participants.
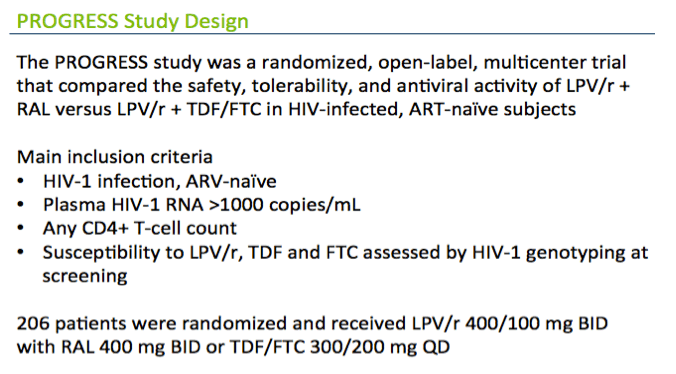
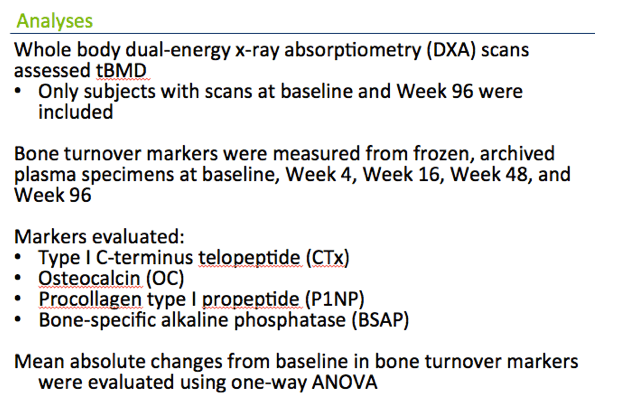
PROGRESS randomized 206 antiretroviral-naive adults to start either raltegravir or TDF/FTC with lopinavir/ritonavir. Seventy-eight people in the raltegravir arm and 82 in the TDF/FTC arm completed the study and had baseline and week-96 DXA scans to measure BMD. At baseline and weeks 4, 16, 48, and 96, researchers measured type 1 C-terminus telopeptide (CTx, a bone resorption marker), osteocalcin, procollagen type 1 propeptide (P1NP), and bone-specific alkaline phosphatase (BSAP) (all bone formation markers). The primary goal was to determine the impact of marker changes on clinically significant bone loss, defined as at least a 5% decrease in total BMD at week 96.
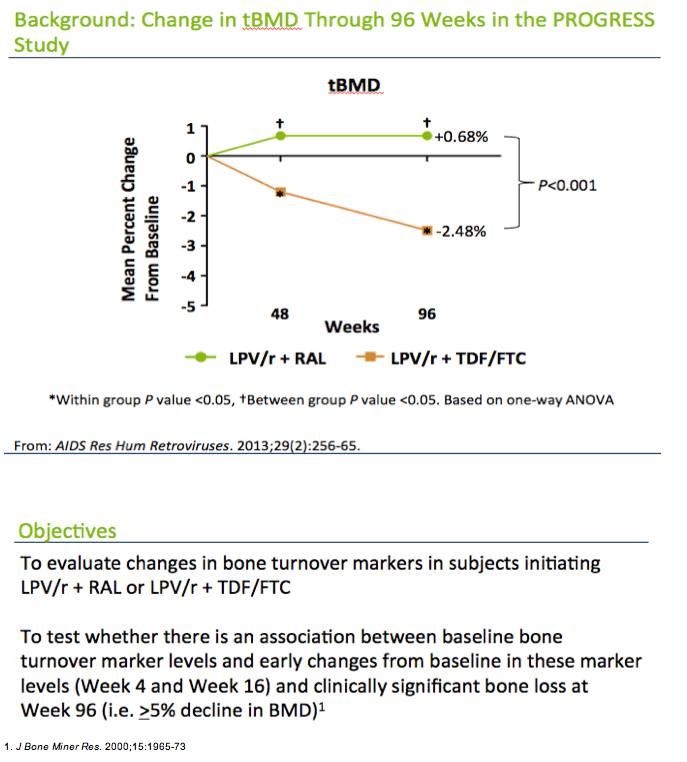
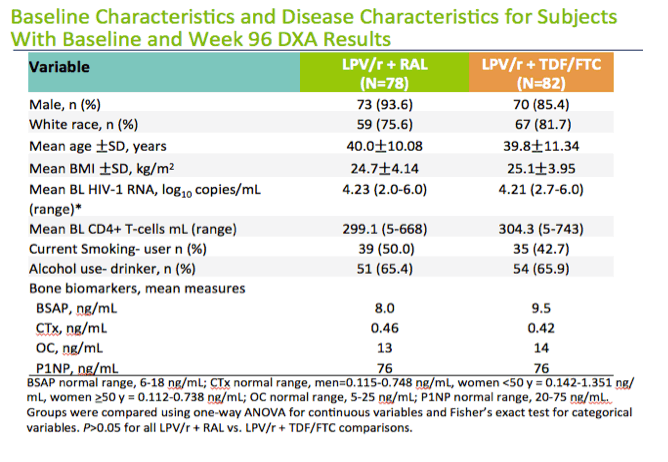
Among people included in the bone turnover analysis, about 90% were men, about 80% were white, and almost half smoked. Age averaged about 40 years in both groups and body mass index about 25 kg/m(2). Two thirds of study participants drank alcohol. Through 96 weeks of treatment, total BMD remained essentially stable in the raltegravir group (+0.68%) while dropping in the TDF/FTC group (-2.48%) (P < 0.001).
Levels of CTx, the resorption (destruction) marker, lay in the mid-normal range at baseline and rose significantly in both treatment arms through 96 weeks. Levels of osteocalcin, a bone formation marker, started in the low-normal range and also climbed significantly in both arms. Levels of P1NP and BSAP lay in the normal range at baseline and did not change much through 96 weeks of treatment in either arm. CTx and osteocalcin levels rose significantly more with TDF/FTC than with raltegravir over 96 weeks.
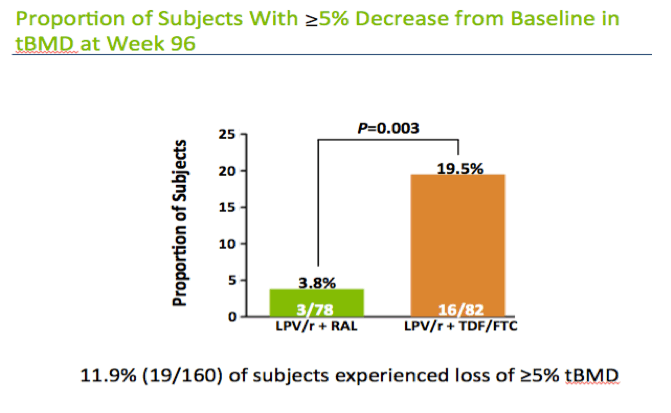
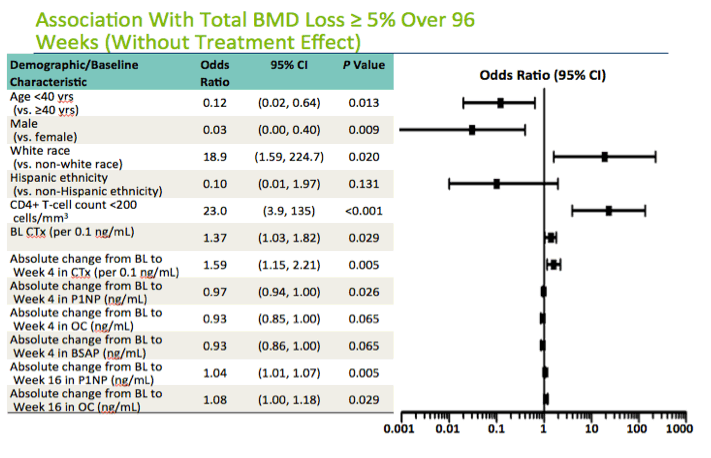
Nineteen of 160 study participants (11.9%) had 5% or greater total BMD loss through 96 weeks, including 3 of 78 in the raltegravir group and 16 of 82 in the TDF/FTC group, a significant difference (3.8% versus 19.5%, P = 0.003). In a logistic regression model that did not include the antiretroviral effect, two nonmarker factors were associated with lower odds of at least a 5% loss in total BMD (age under 40 and male gender) and two nonmarker factors were associated with higher odds of more than 5% bone loss (white race and baseline CD4 count below 200).
In this analysis the impacts of baseline bone marker levels and early bone marker changes were complex. For CTx, the bone resorption marker, higher baseline levels and greater change over 4 weeks were both linked to at least 5% total BMD loss at week 96. But greater week-4 increases in the bone formation markers P1NP, osteocalcin, and BSAP generally protected against 96-week bone loss, although only the P1NP change reached statistical significance. After 16 weeks of treatment, however, larger changes in the bone formation markers P1NP and osteocalcin were positively associated with bone loss, a result suggesting to the investigators that these markers reflect overall bone turnover rather than just bone formation.
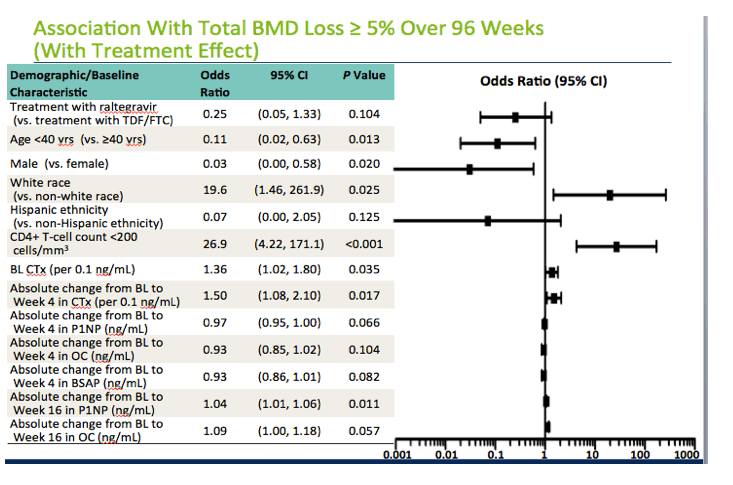
In the analysis including antiretroviral treatment effect, younger age and male gender remained protective against week-96 bone loss of 5% or more, while white race and baseline CD4 count below 200 again raised the odds of week-96 bone loss. And once again higher baseline CTx and greater absolute change in CTx through treatment week 4 were associated with higher odds of total BMD loss at week 96. Associations between baseline bone formation markers and changes at weeks 4 and 16 were all similar to those associations in the analysis excluding treatment effect and approached or achieved statistical significance. In this analysis treatment with raltegravir versus TDF/FTC did not significantly protect against 96-week bone loss, a result suggesting to the investigators that antiretroviral differences in impact on bone loss can largely be attributed to changes in bone turnover markers.
All told, the PROGRESS investigators proposed, "these data provide evidence supporting the hypothesis that early relative changes in markers of bone resorption and bone formation (i.e., the catabolic window) are important predictors of bone loss in HIV-infected persons" starting antiretroviral therapy. They called for further study of the mechanisms behind this effect and the effects of individual antiretrovirals on bone turnover.
References
1. Brown T, Fredrick L, Warren D, et al. Decreased total bone mineral density in treatment-naive subjects taking lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine. 14th European AIDS Conference. October 16-19, 2013. Brussels. Abstract PS7/5.
2. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937-946. http://cid.oxfordjournals.org/content/51/8/937.long
----------------------------------
CHANGES IN BONE TURNOVER MARKERS AND ASSOCIATION WITH DECREASED TOTAL BONE MINERAL DENSITY IN TREATMENT-NA¤VE SUBJECTS TAKING LOPINAVIR/RITONAVIR (LPV/r) COMBINED WITH RALTEGRAVIR (RAL) OR TENOFOVIR/EMTRICITABINE (TDF/FTC)
Reported by Jules Levin
Todd Brown1, Linda Fredrick2, David Warren2, Lim Kai Toh2, Boris Renjifo2, Roger Trinh2, Roula Qaqish2
1: Johns Hopkins University, Baltimore, MD, USA; 2: AbbVie Inc., North Chicago, IL, USA
15th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV
Brussels, Belgium· 16.10.2013
We used total body DXA, which is less sensitive than site-specific DXA and perhaps less clinically relevant

1. AIDS. 2012; 26:2175-2184. 2. AIDS. 2006; 20:2165-2174.
As we have just heard,
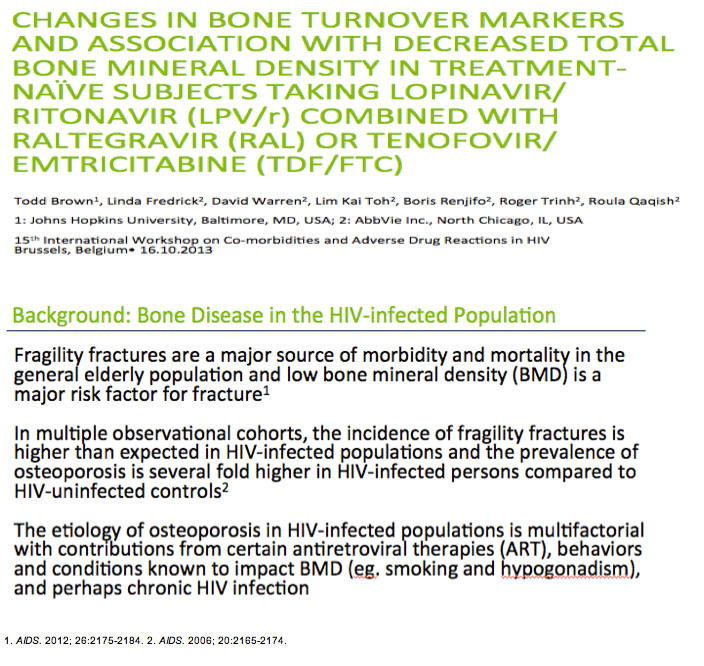
* Fragility fractures are a major source of morbidity and mortality in the general elderly population and low bone mineral density (BMD) is a major risk factor for fracture1
*In multiple observational cohorts, the incidence of fragility fractures is higher than expected in HIV-infected populations and the prevalence of osteoporosis is several fold higher in HIV-infected persons compared to HIV-uninfected controls2
*The etiology of osteoporosis in HIV-infected populations is multifactorial with contributions from certain antiretroviral therapies (ART), behaviors and conditions known to impact BMD (eg. smoking and hypogonadism), and perhaps chronic HIV infection
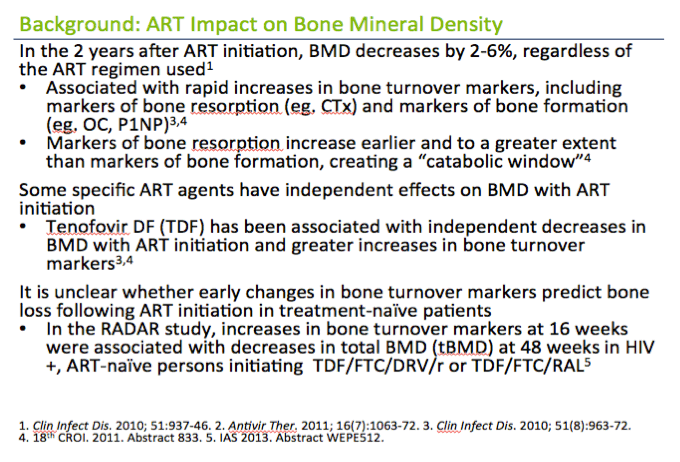
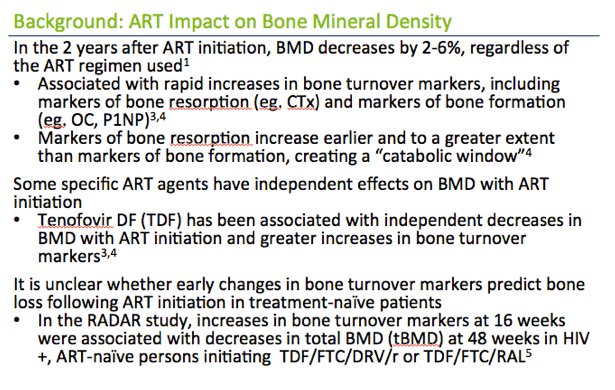
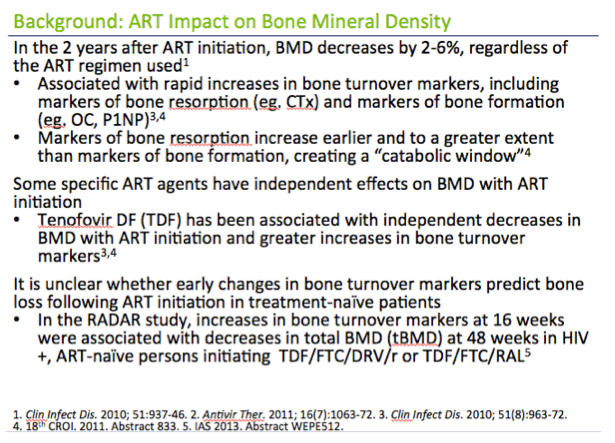
In the 2 years after ART initiation, BMD decreases by 2-6%, regardless of the ART regimen used1
· Similar to the 2-year decline in BMD among women age 50-59 years in the general population1
· Associated with rapid increases in bone turnover markers, including markers of bone resorption (eg. CTx) and markers of bone formation (eg. OC, P1NP)3,4
· Markers of bone resorption increase earlier and to a greater extent than markers of bone formation, creating a "catabolic window"4
Some specific ART agents have independent effects on BMD with ART initiation
· Tenofovir DF (TDF) has been associated with independent decreases in BMD with ART initiation and greater increases in bone turnover markers3,4
It is unclear whether early changes in bone turnover markers predict bone loss following ART initiation in treatment-na´ve patients
· In the RADAR study, increases in bone turnover markers at 16 weeks were associated with decreases in total BMD (tBMD) at 48 weeks in HIV+, ART-na´ve persons initiating TDF/FTC/DRV/r or TDF/FTC/RAL5
The analysis that I'm going to present uses data from the PROGRESS study, which were presented at this meeting 2 years ago in Rome and subsequently published. In this study, ART-na´ve subjects randomized to LPV/r + TDF/FTC had greater loss in BMD compared to those randomized to LPV/r+ RAL . It's interesting to note that this is one of the only studies in the literature looking at bone with ART initiation that has not shown a decrease in BMD in one of its arms.
We used whole body DXA scans to assess tBMD
· Only subjects with scans at baseline and Week 96 were included
· Proportion of subjects with ≥5% decrease from baseline in tBMD at Week 96 was compared between treatment groups
Bone turnover markers were measured from frozen samples at baseline, Week 4, 16, 48, and 96:
We looked at CTx as a marker of bone resoprtion and osteocalcin, P1NP, and bone specific alkaline phosphatase as a marker of bone resorption and we examined the absolute changes in these markers by treatment arm over the 96 weeks.
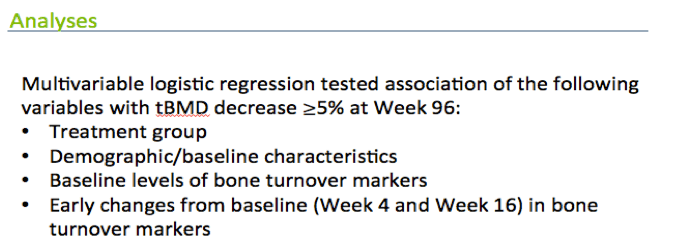
We then used multivariable logistic regression to test whether early changes in bone turnover markers and their baseline levels were associated with bone loss at 96 weeks.
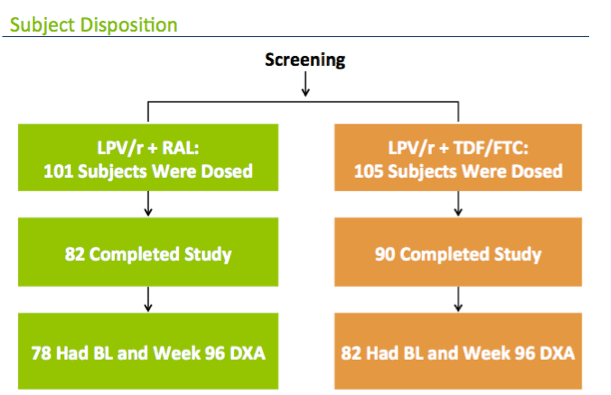
Here's the flow diagram for this analysis: 78 from the raltegravir arm and 82 from the tenofovir arm were included in this analysis
In the LPV/r+RAL arm, of the 101 who were randomized and received at least one dose, 82 completed the study, and 78 had both a baseline and 96 week DXA
In the tenofovir arm, 105 reveived a dose, 90 completed the study, and 82 had DXAs at both timepoints.
The study population was young white males with a normal BMI
Here are box plots of the bone turnover markers at each timepoint by treatment arm. The red dotted lines show the upper and lower bounds of the normal range. As you can see the osteocalcin levels start in the low end of the normal range and then increase quite bit in both arms. The CTX levels start in the mid regions of the normal range and then increase. In contrast, BSAP levels start low and stay low without much change, whereas P1NP starts towards the top of the normal range and stays relatively flat. You can see the changes by arm more clearly on the next slide.
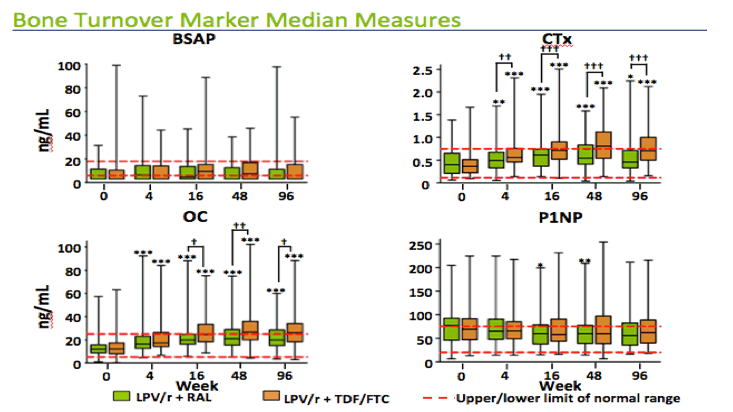
*, **, *** Significant mean change from baseline within a treatment group at P<0.05, 0.01, and 0.001 level, respectively, by one-way ANOVA.
,, Significant difference in mean change from baseline between treatment groups at P<0.05, 0.01, and 0.001 level, respectively, by one-way ANOVA.
This slide shows the absolute changes in both arms over the 96 weeks. Here, you see that osteocalcin increases in both arms, reaching it's peak at 48 weeks, with larger increases in the tenovir/FTC arm. CTX shows a similar pattern but the differences between the arms was even greater.
Interestingly, BSAP and P1NP stayed relatively flat in both arms
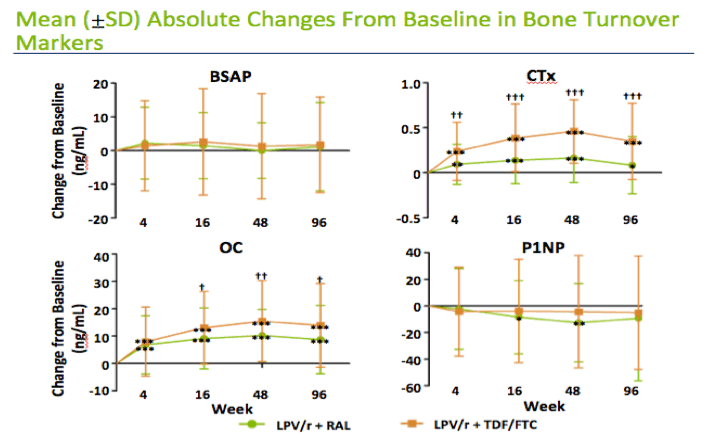
*, **, *** Significant mean change from baseline within a treatment group at P<0.05, 0.01, and 0.001 level, respectively, by one-way ANOVA.
,, Significant difference in mean change from baseline between treatment groups at P<0.05, 0.01, and 0.001 level, respectively, by one-way ANOVA.
This slide shows the proportion of subjects in each arm who had a 5% bone loss at 96 weeks. 16 of the 82 subjects or almost 20% in the tenofovir FTC had this degree of bone loss compared to about 4% in the raltegravir arm.
Here's is the forest plot showing the logistic regression model for 5% or more bone loss over the 96 weeks. We locked the demographic variables into the model and included those variables with a p value of < 0.15 . You see that younger age, male sex were protective against this degree of bone loss, whereas white race and baseline CD4 cell count were associated with bone loss.
The pattern of results for the bone markers and their association with bone loss is interesting. Higher baseline levels of the bone resorption marker CTX and greater changes over 4 weeks were both associated with bone loss at 96 weeks. In contrast, greater increases at 4 weeks in the bone formation marker P1NP, OC, bone specific alkaline phosphatase were generally protective against bone loss, although only the change in P1NP was statistically significant.
By week 16 however, larger changes in these bone formation markers were positviely associated with bone loss, suggesting that at this point these markers are more representative of overall bone turnover, rather than bone formation per se.
This analysis excludes the effect for raltegravir.
Age <40 yrs (vs. ≥40 years), Male (vs. female), White race (vs. non-white race), Hispanic ethnicity (vs. non-Hispanic ethnicity), Alcohol: moderate or heavy drinker/ex-drinker vs. light drinker/ex-drinker or non-drinker, Tobacco: user/ex-user vs. never used, Hepatitis C+ (vs. hepatitis C-), HIV-1 RNA ≥100,000 copies/mL, CD4+ T-cell count <200 cells/mm3, HOMA-IR, BMI (kg/m2), BMD (g/cm2), BL Type I C-terminus telopeptide CTX (per 0.1 ng/mL), BL Procollagen type I propeptide P1NP (ng/mL), BL Osteocalcin OC (ng/mL), BL Bone specific alkaline phosphatase BSAP (ng/mL), Absolute change from baseline to WK4 Type I C-terminus telopeptide CTX (per 0.1 ng/mL), Absolute change from baseline to WK4 Procollagen type I propeptide P1NP (ng/mL), Absolute change from baseline to WK4 Osteocalcin OC (ng/mL), Absolute change from baseline to WK4 Bone specific alkaline phosphatase BSAP (ng/mL), Absolute change from baseline to WK16 Type I C-terminus telopeptide CTX (per 0.1 ng/mL), Absolute change from baseline to WK16 Procollagen type I propeptide P1NP (ng/mL), Absolute change from baseline to WK16 Osteocalcin OC (ng/mL), and Absolute change from baseline to WK16 Bone specific alkaline phosphatase BSAP (ng/mL) were included in a multivariable logistic regression analysis. Those that were significant at the 0.15 level in this analysis were then included in a final multivariable analysis along with the factors of age<40 years, male gender, white race, and Hispanic ethnicity.
Baseline factors independently associated (P<0.05) with reduced incidence of a ≥5% decrease from baseline in total BMD at Week 96 were age <40 years, male gender, and greater absolute change from BL to Wk4 in P1NP. White race, baseline CD4+ T-cell count <200 cells/mm3, higher baseline CTX, and greater absolute change from BL to Wk4 in CTX and from baseline to Wk16 in P1NP and OC were independently associated with increased incidence of a ≥5% decrease from baseline in total BMD at Week 96.
We then repeated the model adding in the treatment effect. What we see is that the treatment effect, which was highly statistically significant in the pervious slide, is no longer statistically significant. This suggests that the differences in the treatment regimens on bone loss can largely be explained by the differences in the changes in markers of bone turnover. The point estimates for the effects of the markers remained unchanged.
"The baseline factors independently associated (P<0.05) with reduced incidence of a ≥5% decrease from baseline in total BMD at Week 96 were male gender and age <40 yrs. White race, baseline CD4+ T-cell count <200 cells/mm3, higher baseline CTX, and greater absolute change from BL to Wk4 in CTX and from baseline to Wk16 in P1NP were independently associated with increased incidence of a ≥5% decrease from baseline in total BMD at Week 96."
|
| |
|
 |
 |
|
|