 |
 |
 |
| |
HCV-TARGET: A Longitudinal, Observational Study of North American Patients with Chronic Hepatitis C Treated with Boceprevir or Telaprevir
|
| |
| |
Reported by Jules Levin
EASL 2013 April 24-28 Amsterdam
Fried MW, Reddy KR, Di Bisceglie AM, Jensen DM, Jacobson IM, Sulkowski M, Terrault N, Afdhal NA, Gordon S, Pockros P, Kwo P, Everson G, Sherman KE, Muir AJ, Pearlman B, Stewart TG, Vainorius M, Peter JA, and Nelson DR for the HCV-TARGET Study Group
Excerpt from NATAP EASL Report by Jurgen Rockstroh:
"One of the raised critical comments towards the CUPIC result is that physician's inexperience with the new HCV compounds may have also affected the safety management and partially may explain the somewhat higher SAE rate observed than in other cohorts..... TARGET cohort which has the aim to evaluate the effects of triple therapy in the broader population now being treated in the United States and Canada, including those underrepresented in clinical trials.......In this first interim analysis, the safety and on-treatment efficacy of telaprevir and boceprevir in the real world setting appeared to be comparable to that observed in registration trials.....despite comparably high rates of hepatic decompensation in the more advanced liver disease patient fortunately very few patients were lost under therapy suggesting that careful monitoring and straight forward AE management can help to minimize therapy associated deaths."
EASL: Summary from EASL 2013 for Hepatitis C - New HCV DAAs on their way soon: what do the phase III studies tell us? - written by - Jurgen K. Rockstroh M.D., Professor of Medicine University of Bonn, Germany - (05/16/13)
ABSTRACT
Phase III trials of boceprevir and telaprevir for HCV provided important information on treatment response, although data is lacking for certain groups, especially those with "difficult to cure" characteristics such as African Americans and cirrhosis. The aim of HCV-TARGET is to evaluate the effects of triple therapy in the broader population now being treated in the United States and Canada, including those underrepresented in clinical trials.
Methods: The HCV-TARGET consortium of academic and community investigators utilizes novel, standardized source data abstraction and a common database to enroll sequential patients treated with regimens that include boceprevir and telaprevir. Demographic, clinical, adverse event, and virological data are collected throughout treatment and post-treatment follow-up. Whole blood for DNA and serum from specified time points are stored at a central biorepository.
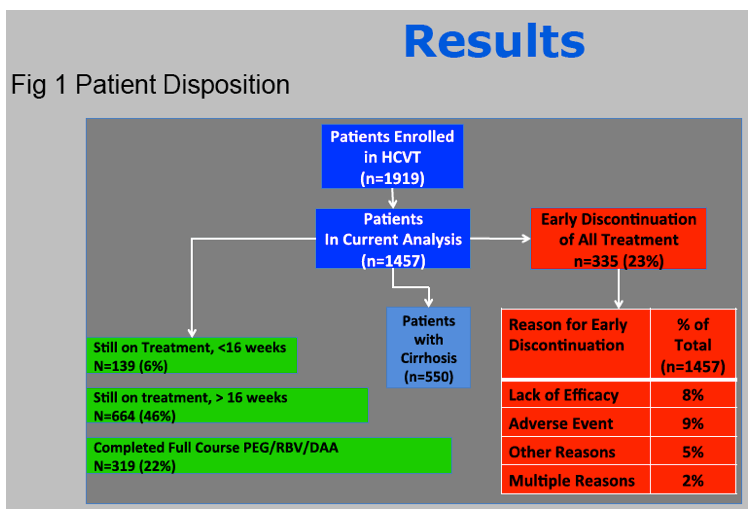
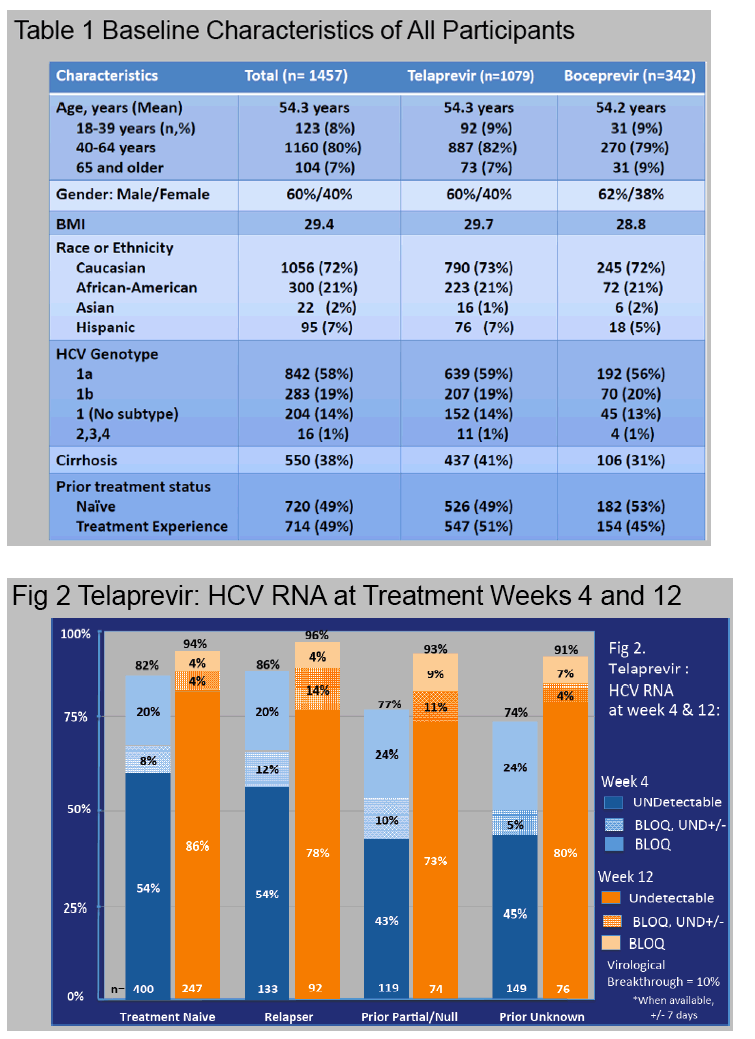
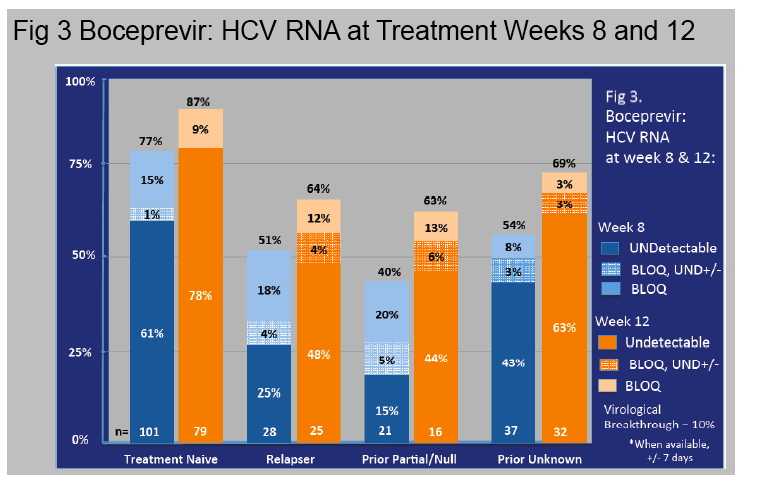
Results: In this ongoing study, 1068 participants have been enrolled to date of whom 816, at varying stages of treatment (telaprevir 77%, boceprevir 23%), are included in this preliminary analysis. The majority are male (60%), Caucasian (76%), and between ages 40-64 years (84%).
African Americans comprised 20% of participants and 7% of patients were older than 65 years.
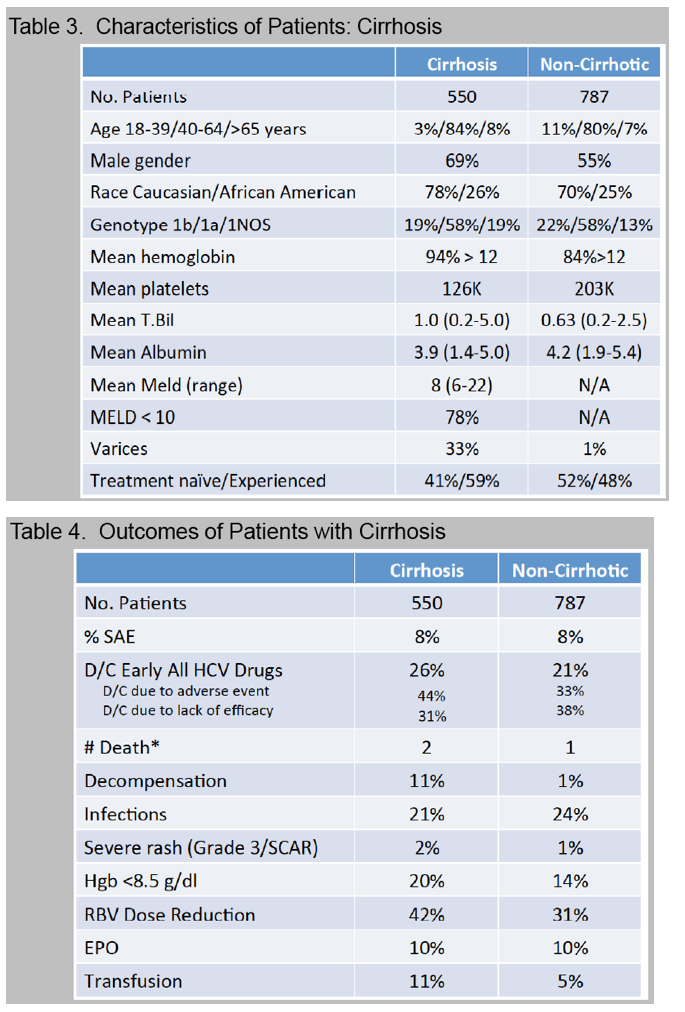
Cirrhosis, defined by biopsy or clinical criteria, was present in 35%.
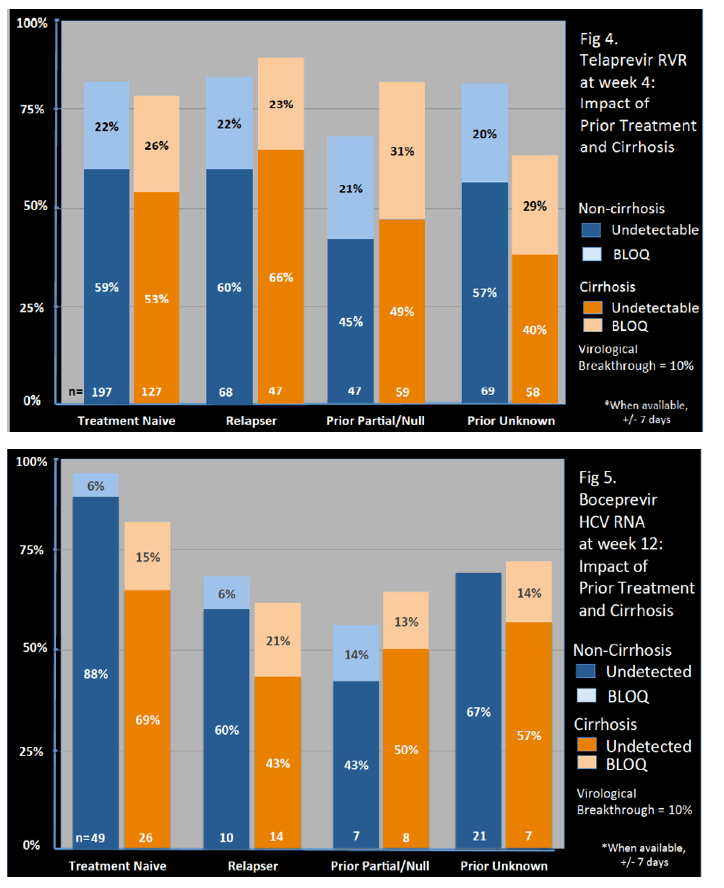
Genotype 1a/1b/not subtyped was 61%, 22%, 11%, respectively, and 49% were treatment na´ve.
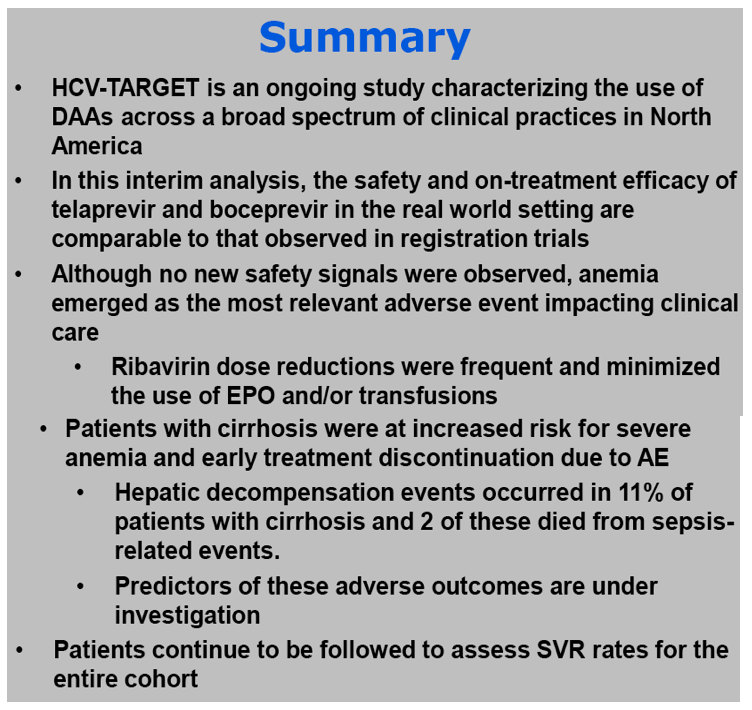
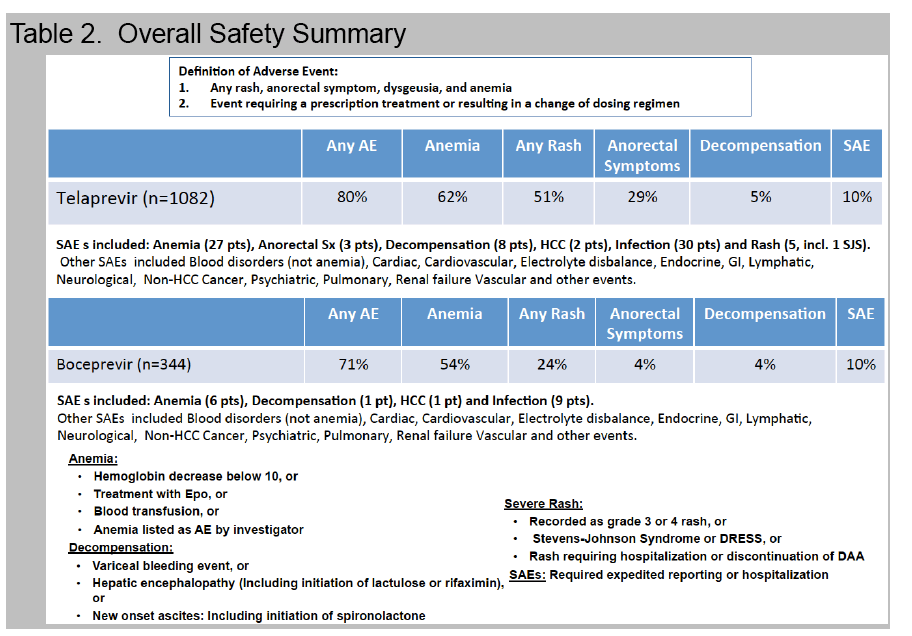
Adverse events (AE) requiring medical intervention or dose modification to the antiviral regimen occurred in 76%.
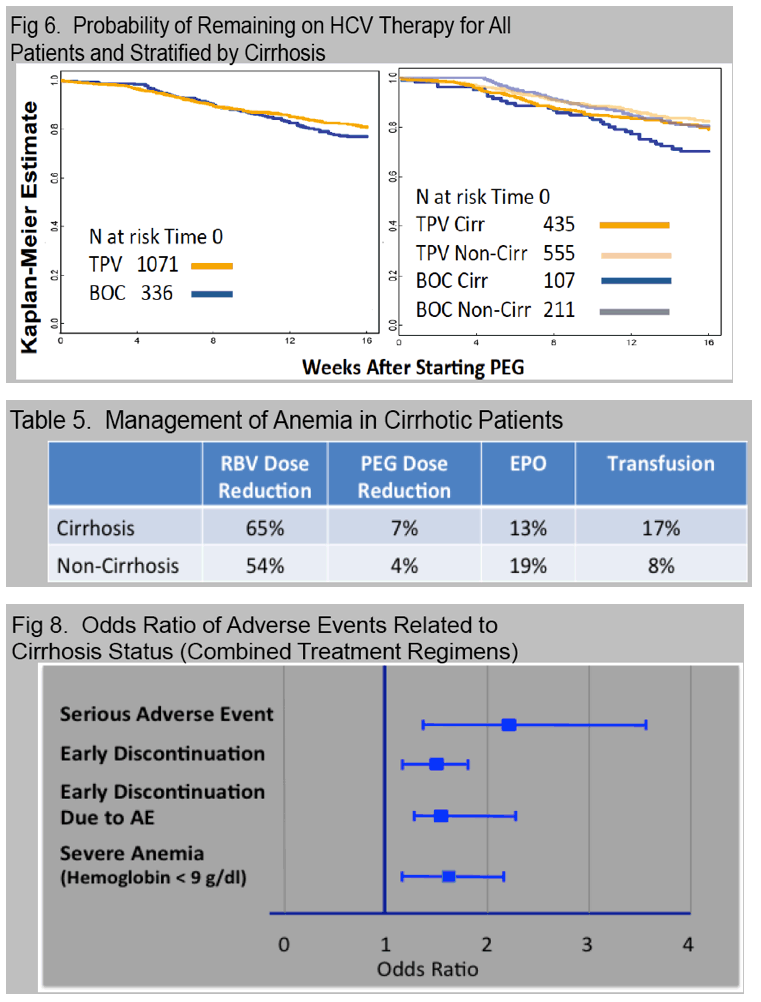
Anemia, occurring in 56% with nadir hemoglobin < 8.5 g/dl (12%) or 8.5-10 g/dl (26%), was managed with ribavirin dose reduction (45%), EPO (15%), and/or transfusion (8%).
Rash was noted in 29% while 1% discontinued treatment due to rash.
A new decompensating event (ascites, encephalopathy, variceal hemorrhage) occurred in 11 (4%) of cirrhotic patients.
Treatment was prematurely discontinued due to AE in 5% of non-cirrhotic and 10% of cirrhotic patients. Data collection is ongoing and SVR data will be presented.
Conclusions: HCV-TARGET encompasses the North American experience with boceprevir and telaprevir across a broad spectrum of clinical practices in a cohort enriched with African American patients and cirrhosis. Continuing analyses will inform clinicians regarding these previously underrepresented populations and best practices for managing adverse events.


|
| |
|
 |
 |
|
|