| |
Pre & Post Liver Transplant Treatment- Recent Studies - New DAAs, Boceprevir, Telaprevir - "Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: A multicenter experience" and Editorial
|
| |
| |
Download the PDF here
-----------------------------------------------
Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: A multicenter experience - (12/27/13)
Sofosbuvir and Daclatasvir Combination Therapy in a Liver Transplant Recipient With Severe Recurrent Cholestatic Hepatitis C - (11/25/13)
Drug-drug interactions with oral anti-HCV agents and Idiosyncratic Hepatotoxicity in the Liver Transplant setting - (11/25/13)
AASLD: Sustained Virological Response After Protease Inhibitor-based Therapy For Hepatitis C Recurrence After Liver Transplantation: A Multicentric European Experience - (11/18/13)
AASLD: Pretransplant Sofosbuvir and Ribavirin to Prevent Recurrence of HCV Infection After Liver Transplantation - (11/06/13)
AASLD: Sofosbuvir and Ribavirin for the Treatment of Established Recurrent Hepatitis C Infection After Liver Transplantation: Preliminary Results of a Prospective, Multicenter Study - (11/05/13)
AASLD: Initial Evaluation of the Sofosbuvir Compassionate Use Program for Patients With Severe Recurrent HCV Following Liver Transplantation - (11/06/13)
----------------------------------------------
Editorial
Victory and defeat at Heraclea - Treating hepatitis C infection following liver transplantation with telaprevir and boceprevir
Journal of Hepatology
January 2014
HCV associated liver disease continues to be the most common indication for liver transplantation in the West. Although the impact of HCV infection varies substantially between recipients, allograft failure secondary to recurrence of HCV infection is the most frequent cause of death and graft failure in HCV infected recipients. Attenuating the impact of HCV on posttransplant patient and graft survival has been a critical priority for transplant physicians and their patients. In this edition of the Journal of Hepatology Duclos-Vallee et al., report the results of a multicenter study of 37 liver transplant recipients (male: 92%, age 57 ± 11 years), who were treated with PEG interferon, ribavirin and boceprevir (n = 18) or telaprevir (n = 19) for recurrence of HCV infection following liver transplantation. The indication for therapy was progressive HCV recurrence (fibrosis stage F2 (83%) or fibrosing cholestatic hepatitis (16%)). Eighteen patients were treatment-naive, five were relapsers and 14 were non-responders to prior dual therapy after LT. The patient population was, by and large, typical of recipients with post-LT HCV infection who are considered for boceprevir and telaprevir based antiviral therapy. The main finding of the study by Duclos-Vallee et al., is that a sustained virological response (SVR) at 12 weeks after treatment discontinuation was observed in 20% and 71% of patients in the telaprevir (TVR) and boceprevir (BOC) groups, respectively, for an overall SVR rate of 50%. While a study with an n of 37 may seem unimportant, to dismiss the results of this study would be to miss an opportunity to gain insights into the opportunities and challenges of treating posttransplant HCV infection. The report by Duclos-Vallee et al., which is thoughtful in design and presentation, is not without limitations, including small sample size, lack of randomization and absence of a prospective antiviral treatment protocol. To focus on the shortcomings would, however, be a disservice to the field and the authors. There are important lessons to be had. The first and most obvious lessons are that boceprevir and telaprevir are neither particularly effective nor safe in this patient population. While the authors focus on the "encouraging" complete early virological response rate (cEVR) of 73%, when it comes to treating HCV, particularly following liver transplantation, SVR is king. The observed SVR rate of 50% may be viewed as somewhat less encouraging than the cEVR rate. The benefit of treatment that may theoretically eventually be reaped by those recipients who achieved SVR needs to be weighed against the cost of boceprevir and telaprevir based antiviral therapy. Three patients (8%) died on treatment. With only 28 (of 37) patients reaching the combined endpoints of end of treatment/death/treatment discontinued, nine patients are still on treatment. Plenty of opportunity for participants to die and experience non-lethal adverse events thus remains. The non-lethal adverse events are of particular interest. Deterioration in renal function was common, with 5 (14%) of the patients who survived antiviral treatment developing renal failure, with a mean decline in GFR of 3.8 ml/min during treatment. As renal function is one of the best predictors of longterm outcomes, the negative impact of antiviral therapy may continue well beyond the end of antiviral treatment. Add in one third of patients getting hospitalized for sundry other adverse events and one third requiring blood transfusions and the net benefit is not completely obvious. Only half of five year posttransplant mortality/graft loss is due to HCV recurrence. Overall five year survival rates for recipients with HCV infection are ~70% and the risk of mortality related specifically to HCV recurrence is 15% by the fifth postoperative year [1]. A 100% SVR could thus reduce 5 year post-LT mortality by 15%. With an observed mortality rate related to antiviral treatment in the study by Duclos-Vallee et al., of 8%, the best case net benefit is a 7% reduction in five year mortality attributable to antiviral therapy. As two thirds of those with the most severe recurrence of HCV did not respond to antiviral therapy in this study, it is entirely plausible that there will be no net survival benefit to treating liver transplant recipients with BOC or TVR in the medium term (the likelihood of mortality/graft loss due to HCV is likely to be highest among the FCH/cirrhosis patients, who had a low SVR rate). The frequency of SAEs greatly limited the potential efficacy posttransplant antiviral therapy in the study by Duclos-Vallee et al., with only half of the ~50% of patients who discontinued treatment doing so for virological nonresponsiveness/breakthrough, the remainder dying or experiencing adverse events severe enough to stop antiviral treatment. A third lesson of the study by Duclos-Vallee et al., is that full realization of the potential benefit of BOC and TVR based posttransplant antiviral therapy requires minimisation of the side effects of these agents and those of peginterferon and ribavirin. The high frequency of infections and renal insufficiency suggests overexposure to calcineurin inhibitors. This is despite effective CNI trough level management through dose adjustments in anticipation of in response to the introduction of the cytochrome P450 inhibitors boceprevir and telaprevir. Renal insufficiency and life threatening infections despite stable CNI levels is a consistent emerging theme of posttransplant antiviral therapy. A thorough appreciation of the impact of post-LT antiviral therapy on the pharmacokinetics of immunosuppression agents is essential to achieving optimal safety and efficacy.
The cytochrome P450 (CYP) enzyme system is responsible for drug metabolism via oxidation in the liver and intestines allowing drugs to be eliminated into the bile or urine. The CYP 3A4 isoenzyme is used by more than 50% of approved medications for elimination from the body [1]. Protease inhibitors, such as boceprevir (BOC) and telaprevir (TVR), in addition to being potent inhibitors of the CYP 3A4 enzyme leading to many potential drug-drug interactions (DDI), are also (TVR >BOC) inhibitors of P-gp, the active transport enzyme, p-glycoprotein (P-gp). P-gp is an efflux pump that ultimately inhibits intestinal absorption of medications from the gastrointestinal tract. Inhibition of P-gp can increase concentrations of drugs that would typically be blocked from absorption into the blood stream. Examples of medications that will be increased during coadministration of TVR via P-gp inhibition are morphine [2], digoxin [3] and midazolam [3] (Table 1). The onset of CYP 3A4 inhibition is typically within the first couple of days of protease inhibitor therapy. BOC and TVR exhibit mechanism-based inhibition of CYP 3A4 meaning that the isoenzyme is inhibited for its life until new CYP 3A4 protein can be synthesized. The corollary is that resolution of CYP 3A4 inhibition may be delayed after discontinuing protease inhibitor therapy. Empiric dose adjustments, judicious monitoring of co-administered drugs, and screening for potential adverse effects are warranted during and after BOC and TVR initiation and discontinuation.
Table 1. Empiric recommended dosing strategies of concomitant protease inhibitors and immunosuppressants when both initiating and discontinuing protease inhibitor therapy. Empiric dose changes should be done in conjunction with therapeutic drug monitoring.
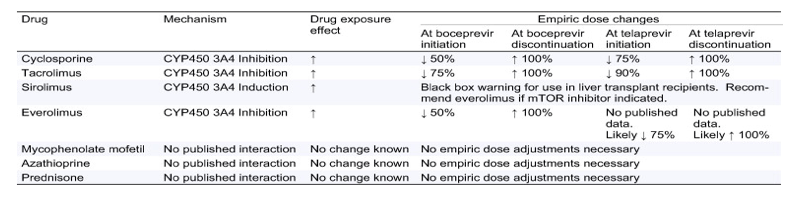
Most available data for drug-drug interactions (DDI) with these protease inhibitors are in healthy volunteers with normal hepatic function. As HCV can decrease CYP function, increasing calcineurin inhibitor concentrations by approximately 30% [4], [5], pharmacokinetic effects in LT recipients with HCV infection may be more pronounced than those seen in healthy volunteers.
Tacrolimus doses as little as 0.5 mg per week are adequate to maintain therapeutic concentrations when given with the protease inhibitor combination of lopinivir/ritonavir [6]. In healthy volunteers TVR can increase tacrolimus concentrations as much as 70-fold and cyclosporine concentrations 4.6-fold [7], while BOC increases tacrolimus concentrations 17-fold and cyclosporine 2.7 fold [8]. Based on the known effects of BOC and TVR, CNI and mTOR doses should be decreased empirically when starting protease inhibitor therapy and consequently increased when protease inhibitor therapy is discontinued. Sirolimus, and everolimus are also known substrates of CYP 3A4 and P-gp. No published data exist describing the DDI between everolimus and TVR, however everolimus clearance is decreased by 52%, when administered with BOC [9]. As sirolimus carries a black box warning for use in liver transplantation [10], it may be wise to avoid this agent altogether in patients receiving TVR or BOC. Consideration might be given to everolimus use in place of sirolimus if an mTOR inhibitor is indicated. The shorter half-life of everolimus may make management of drug-drug interactions easier than sirolimus.
DDI can be significant in transplant recipients as the calcineurin inhibitors, mTOR inhibitors, and a multitude of other medications are transported by P-gp and/or metabolized by the CYP 3A4 enzyme. It is important to screen concomitantly administered medications other than the CNIs and mTORs for potential DDI or contraindications. Common CYP 3A4 substrates include azole antifungal agents, HMG-Co-A reductase inhibitors (statins), methadone, and many others. Increasing the frequency of therapeutic drug monitoring of immunosuppressants and other concomitant medications is imperative when both starting and stopping protease inhibitors.
Finally, as all CNI trough levels are measured in whole blood, trough levels will not accurately reflect the biologically active (immunosuppressive and nephrotoxic) free CNI trough levels. The major portion of whole blood CsA and TAC is sequestered in erythrocytes, with hematocrit known to be inversely related to plasma concentrations of CNIs [11]. Due to RBV induced hemolysis a shift of the erythrocyte-bound CsA fraction to plasma will occur. Anemia will be exacerbated by peginterferon and BOC induced bone marrow suppression. In the context of progressive, ubiquitous and frequently severe anemia relying on whole blood level monitoring may not be safe. As free CNI level monitoring is not widely available, consideration should be given to adjusting target CNI whole blood trough levels downward in the context of a falling hematocrit.
On being congratulated for his victory over the Romans at Heraclea, King Pyrrhus, whose army had suffered irrecoverable casualties, replied that one more such victory would utterly undo him. The report by Duclos-Vallee et al., should serve to remind us of the possibly Pyrrhic nature of our battle with posttransplant HCV infection. We eagerly await the advent of HCV therapies that are more effective and more easily tolerated than those that incorporate BOC and TVR. For patients with mild recurrence, waiting may be more prudent than joining the battle.
|
|
| |
| |
|
|
|