| |
Sangamo: Maintained Reduction of Viral Load at or Below Limit of Detection Ongoing at 14 Weeks, Data were presented at the Annual Meeting of the European Society of Gene and Cell Therapy (ESGCT and SETGyC Collaborative Congress) which is being held in Madrid from October 25-28, 2013
|
| |
| |
(see slides below)
Sangamo BioSciences, Inc. (Nasdaq: SGMO) announced today the presentation of new data demonstrating sustained control of HIV viral load (VL) at or below the limit of detection for 14 weeks (at last measurement) in an SB-728-T- treated HIV-infected subject who was not on antiretroviral therapy (ART). The CCR5 delta-32 heterozygote subject is enrolled in Sangamo's clinical trial (SB-728-902 Cohort 5) and, as part of the clinical trial protocol, is undergoing an ART treatment interruption (TI), which is ongoing.
Summary
SB-728-T Infusion in CCR5 Δ32 Heterozygote HIV-infected Subjects
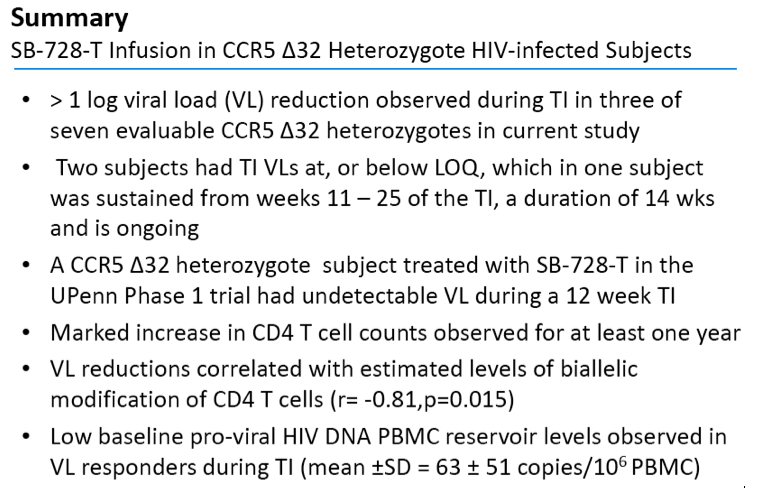
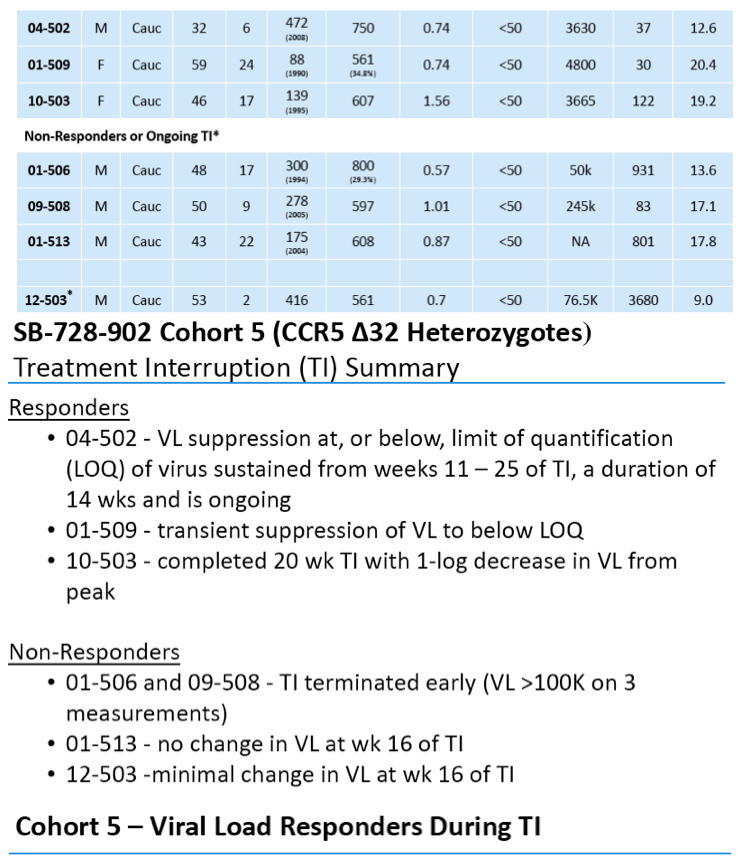
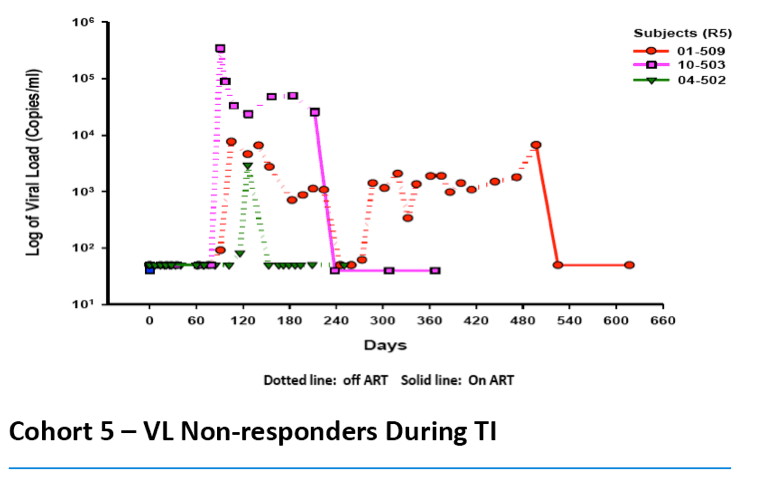
·> 1 log viral load (VL) reduction observed during TI in three of seven evaluable CCR5 Δ32 heterozygotesin current study
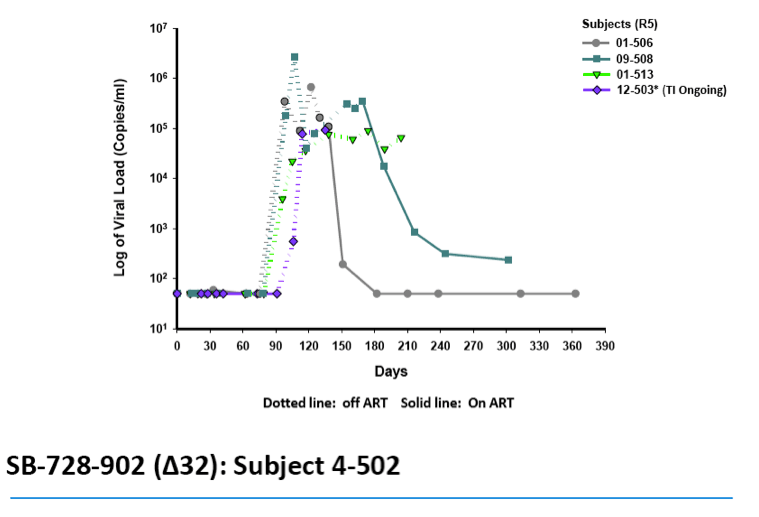
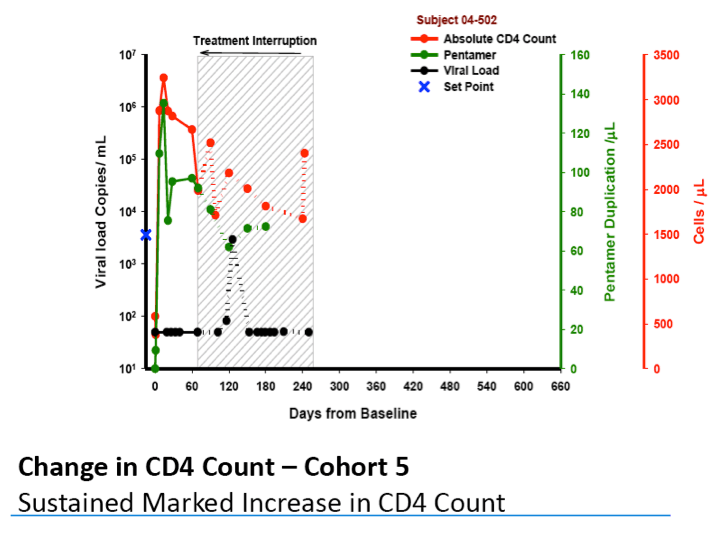
·Two subjects had TI VLs at, or below LOQ, which in one subject was sustained from weeks 11 Š25 of the TI, a duration of 14 wks and is ongoing
·A CCR5 Δ32 heterozygote subject treated with SB-728-T in the UPennPhase 1 trial had undetectable VL during a 12 week TI
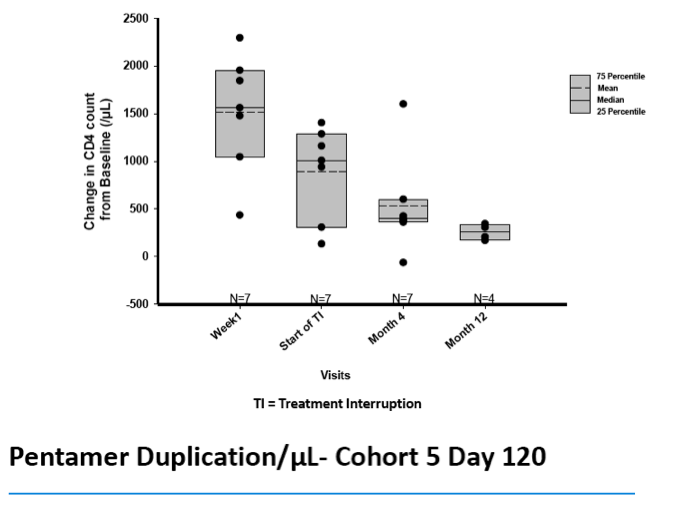
·Marked increase in CD4 T cell counts observed for at least one year
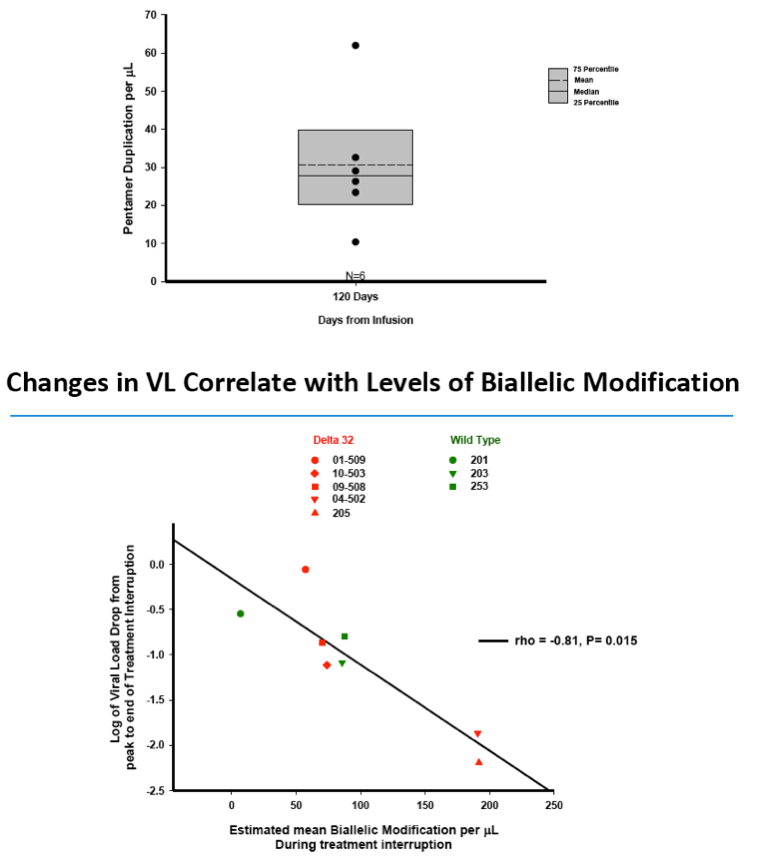
·VL reductions correlated with estimated levels of biallelicmodification of CD4 T cells (r= -0.81,p=0.015)
·Low baseline pro-viral HIV DNA PBMC reservoir levels observed in VL responders during TI (mean ±SD = 63 ±51 copies/106 PBMC)
Conclusions
SB-728-T Infusion in CCR5 Δ32 Heterozygote HIV Subjects
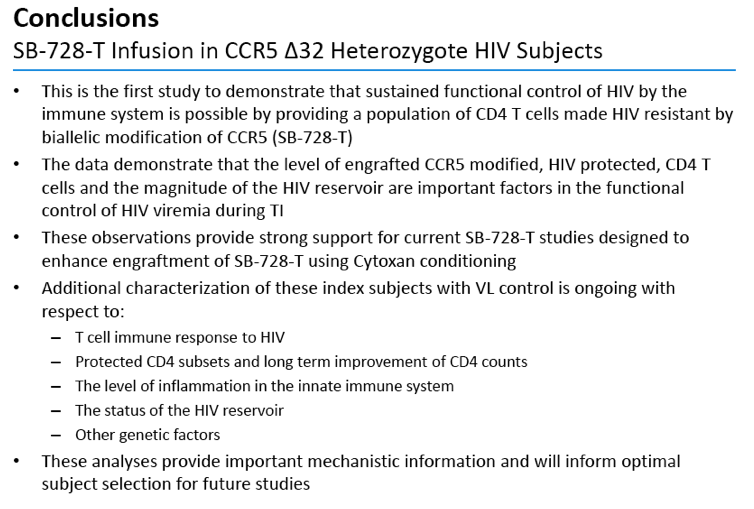
·This is the first study to demonstrate that sustained functional control of HIV by the immune system is possible by providing a population of CD4 T cells made HIV resistant by biallelicmodification of CCR5 (SB-728-T)
·The data demonstrate that the level of engrafted CCR5 modified, HIV protected, CD4 T cells and the magnitude of the HIV reservoir are important factors in the functional control of HIV viremiaduring TI
·These observations provide strong support for current SB-728-T studies designed to enhance engraftment of SB-728-T using Cytoxanconditioning
·Additional characterization of these index subjects with VL control is ongoing with respect to:
ŠT cell immune response to HIV
ŠProtected CD4 subsets and long term improvement of CD4 counts
ŠThe level of inflammation in the innate immune system
ŠThe status of the HIV reservoir
ŠOther genetic factors
·These analyses provide important mechanistic information and will inform optimal subject selection for future studies
Reduction of Viral Load at or Below Limit of Detection Ongoing at 14 Weeks (see slides below)
Additional Presentations of Preclinical Data at Annual Meeting of European Society of Gene and Cell Therapy (ESGCT)
RICHMOND, Calif., Oct. 28, 2013 /PRNewswire/ -- Sangamo BioSciences, Inc. (Nasdaq: SGMO) announced today the presentation of new data demonstrating sustained control of HIV viral load (VL) at or below the limit of detection for 14 weeks (at last measurement) in an SB-728-T- treated HIV-infected subject who was not on antiretroviral therapy (ART). The CCR5 delta-32 heterozygote subject is enrolled in Sangamo's clinical trial (SB-728-902 Cohort 5) and, as part of the clinical trial protocol, is undergoing an ART treatment interruption (TI), which is ongoing.
Data were presented at the Annual Meeting of the European Society of Gene and Cell Therapy (ESGCT and SETGyC Collaborative Congress) which is being held in Madrid from October 25-28, 2013.
"These data demonstrate that sustained functional control of HIV in the absence of ART is possible with a single SB-728-T treatment," stated Geoff Nichol, M.B., Ch.B., Sangamo's executive vice president of research and development. "Our aim is to provide a population of immune memory cells that are protected from HIV infection and are capable of generating an effective immune response against the virus throughout the body. These data represent a further step toward demonstrating the efficacy and durability of this therapeutic approach."
Dr. Nichol added, "We continue to follow these Cohort 5 subjects and look forward to presenting a complete data set from this study, and a second ongoing trial (SB-728-1101), designed to maximize the engraftment of SB-728-T in subjects who are not CCR5 delta-32 heterozygotes, later this year."
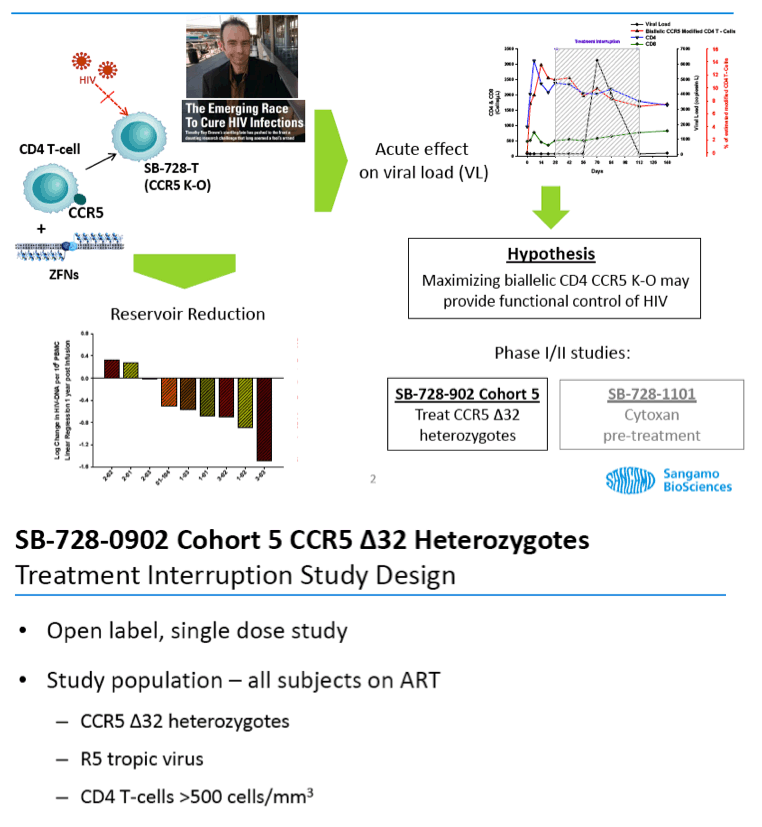
Data from Sangamo's Phase 1 and 2 studies demonstrate that VL became undetectable during a TI from ART in three of seven evaluable CCR5 delta-32 heterozygote HIV-infected subjects, including two of six subjects that had completed TI in the ongoing SB-728-902 Cohort 5 study and an additional CCR5 delta-32 heterozygote subject from an earlier Phase 1 clinical trial of SB-728-T. In one SB-728-902 Cohort 5 subject, VL has remained undetectable (at or below the limits of quantification of the current ultra-sensitive assays for HIV) for 14 weeks (to last measurement taken) and the TI is ongoing. Reduction in VL from peak during TI showed a statistically significant correlation (p=0.015) with estimated numbers of engrafted ZFN modified cells (SB-728-T) in which both copies of the CCR5 gene had been disrupted (biallelic modification), in line with previously presented data from this program.
Collectively, data from these studies demonstrate that in all trial subjects, SB-728-T treatment results in a durable increase in total CD4 T-cells. In addition, seven of nine subjects enrolled in Sangamo's Phase 1 study (SB-728-902 Cohorts1-3) experienced a longer term reduction in the viral reservoir as measured by HIV DNA in peripheral blood mononuclear cells, a source of chronic HIV infection not addressed by current ART.
Sangamo scientists and collaborators also presented data from preclinical and research programs in hemophilia and lysosomal storage disorders, hemoglobinopathies, cancer, cystic fibrosis, immunodeficiencies and the application of ZFN-mediated CCR5 modification in stem cells for HIV.
"The presentations at the ESGCT meeting demonstrate the diversity and breadth of potential therapeutic applications of Sangamo's ZFP technology," said Edward Lanphier, Sangamo's president and CEO. "We look forward to continuing to update on our progress in the coming months at a translational medicine meeting organized by The Lancet entitled, 'What Will it Take to Achieve an AIDS-free World?' in San Francisco, from November 3-5, as well as at the Sixth International Workshop on HIV Persistence, Reservoirs & Eradication Strategies in Miami in early December. In addition, we will present data from Sangamo's preclinical programs at the Annual Meetings of the Society for Neuroscience in November and the American Society of Hematology (ASH) in early December."
Summary of Clinical Trial Design
About SB-728-902 Cohort 5
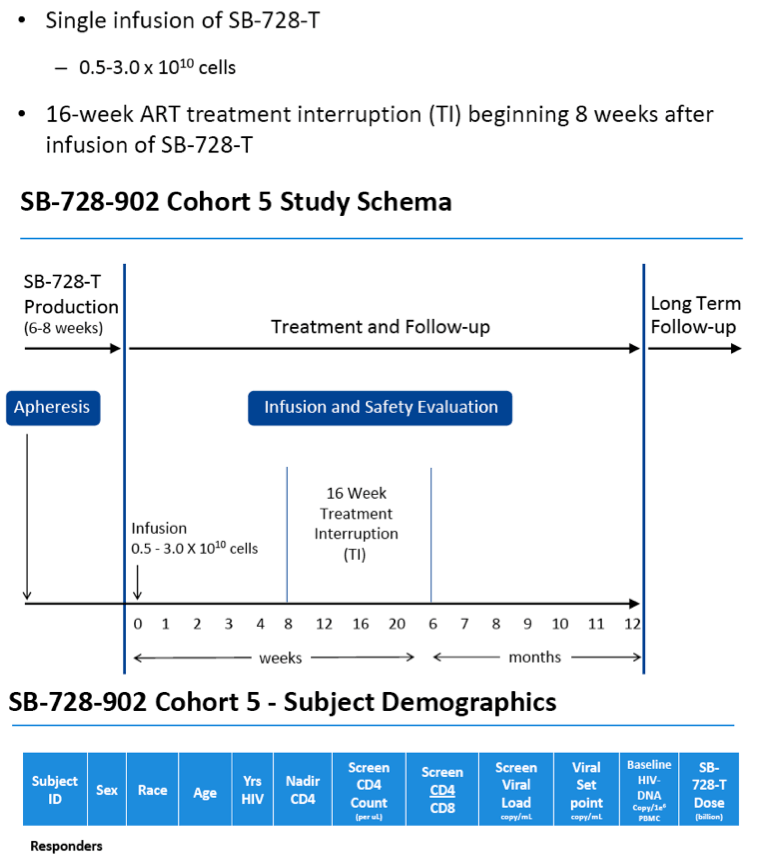
Ten HIV-infected subjects heterozygous for the CCR5 delta-32 mutation (i.e. with one CCR5 gene that is naturally modified) who are currently on ART have been enrolled and have received a single intravenous infusion of SB-728-T (5 to 30 billion modified cells). Two months after SB-728-T treatment, subjects undergo a 16 week TI during which time their ART is discontinued. ART is reinstituted in subjects whose CD4 T-cell counts drop to <350 cells/ mm(3) and/or whose HIV-RNA increases to >100,000 /mL for three consecutive weekly measurements. At the end of the TI, subjects with a sustained detectable HIV viral load are reinstituted on ART. Subjects with an undetectable viral load can remain off ART until HIV RNA levels are detectable or their CD4 T-cell count drops below 350 cell/mm(3) for three consecutive weekly measurements.
A total of ten subjects have been treated in this cohort.
Of the six evaluable subjects, we observed two subjects in which their VL became undetectable during TI from ART:
-- In one subject, VL suppression at, or below, the limit of quantification
(LOQ) of virus was sustained from week 11 -- 25 of TI and the TI is
ongoing.
-- In the second subject there was a transient suppression of VL at or below
LOQ.
-- A third subject completed the TI with 1-log decrease in VL from peak.
In three subjects, there was no reduction in VL during the TI, one completed the TI and in two the TI was terminated early due to their viral loads exceeding the upper limit allowed in the protocol. A seventh subject has not completed TI and is still being evaluated.
About SB-728-902 Cohorts 1-3
The study is an open-label Phase 1 clinical trial to evaluate the safety and tolerability of single infusions of an escalating dose of an autologous (a patient's own) CD4+ T-cell product genetically modified at the CCR5 gene by CCR5-specific ZFNs (SB-728-T). The trial enrolled nine HIV-infected subjects (three cohorts of three subjects each) who have sub-optimal T-cell levels and no detectable viral load on long-term ART. Subjects remained on their existing antiviral therapy while receiving treatment with SB-728-T.
About SB-728-T
Sangamo's drug, SB-728-T, is generated by ZFN-mediated modification of the gene encoding the CCR5 receptor in a patient's own T-cells. ZFN modification disrupts the expression of this key co-receptor for HIV entry and renders cells resistant to HIV infection. The approach is based on the observation that a naturally occurring mutation in the CCR5 gene, CCR5 delta-32, provides protection from HIV infection. Individuals in whom both copies of the CCR5 gene carry the delta-32 mutation are generally not susceptible to the most common strain of HIV.

|
|
| |
| |
|
|
|