 |
 |
 |
| |
New HIV 'Cure' Drug To Activate HIV Replication- Cyclic Panobinostat (LBH589) dosing in HIV-1 patients: Findings from the CLEAR trial
|
| |
| |
Reported by Jules Levin
IAS 2013 Kuala Lumpur June 30-July 3
Martin Tolstrup
Aarhus University Hospital, Denmark
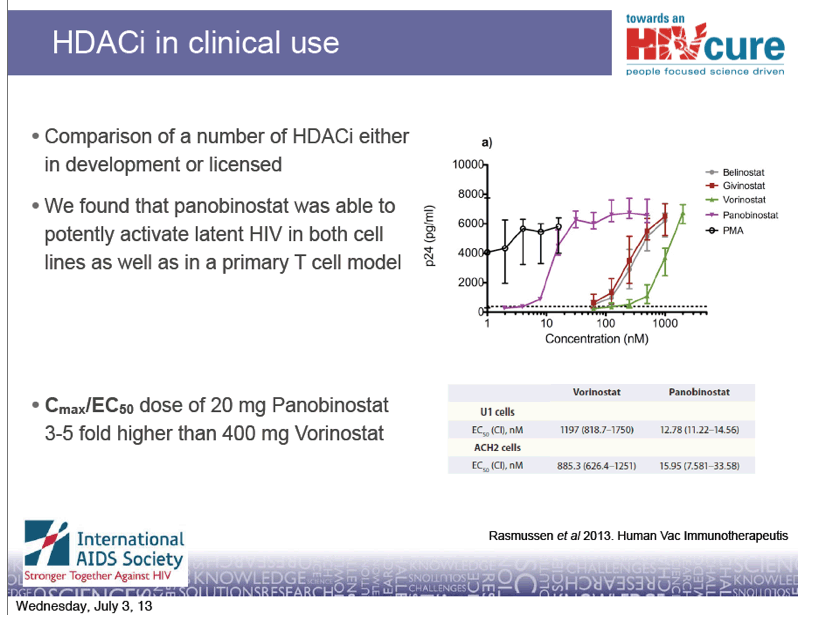
· Panobinostat is a cinnamic hydroxamic acid analogue very
similar to Vorinostat
· Panobinostat is being developed by Novartis for treatment of
Multiple myeloma (Phase III) and Acute Myeloid Leukemia
(Phase II)
· Panobinostat is described as a Pan-HDAC inhibitor with
inhibitory activity in the lower nM range
· Considerable inhibitory activity against HDAC 1, 2 and 3
which appear important for disruption of HIV latency
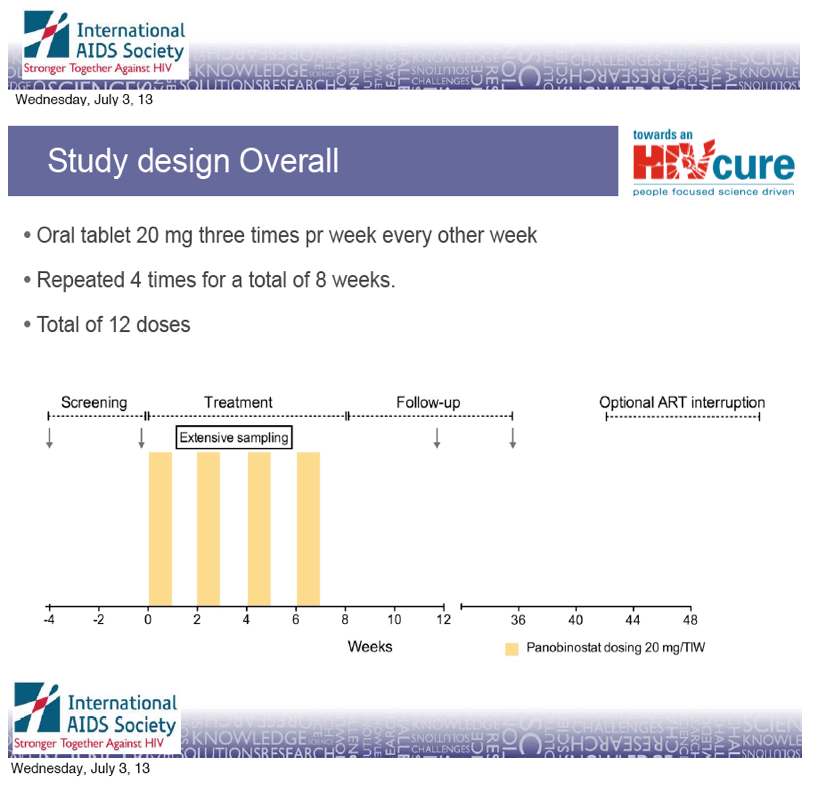
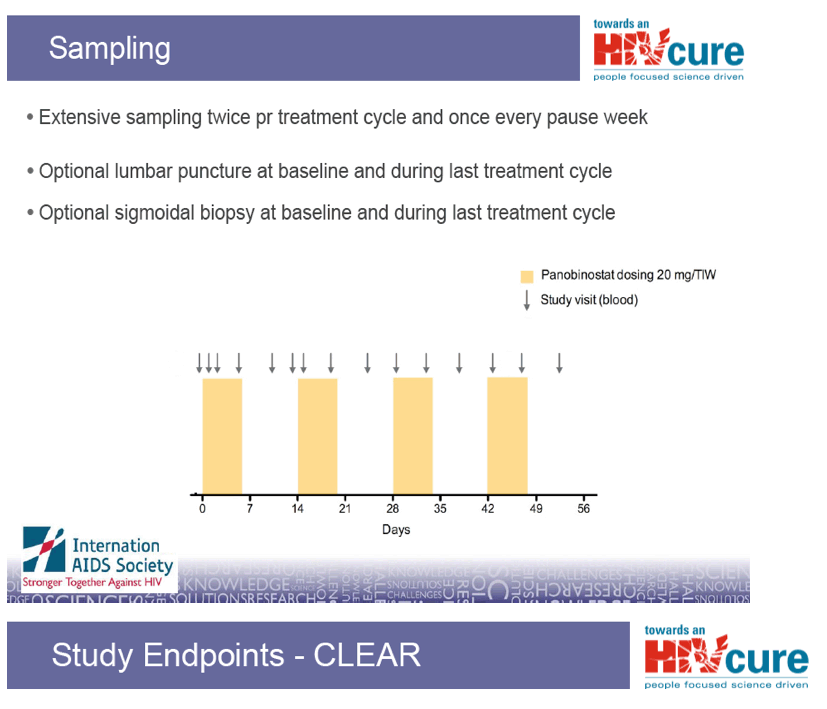
Oral tablet 20 mg three times pr week every other week
· Repeated 4 times for a total of 8 weeks.
· Total of 12 doses
Would 20 mg oral Panobinostat dosing lead to viral reactivation in virological
suppressed HIV-1 patients?
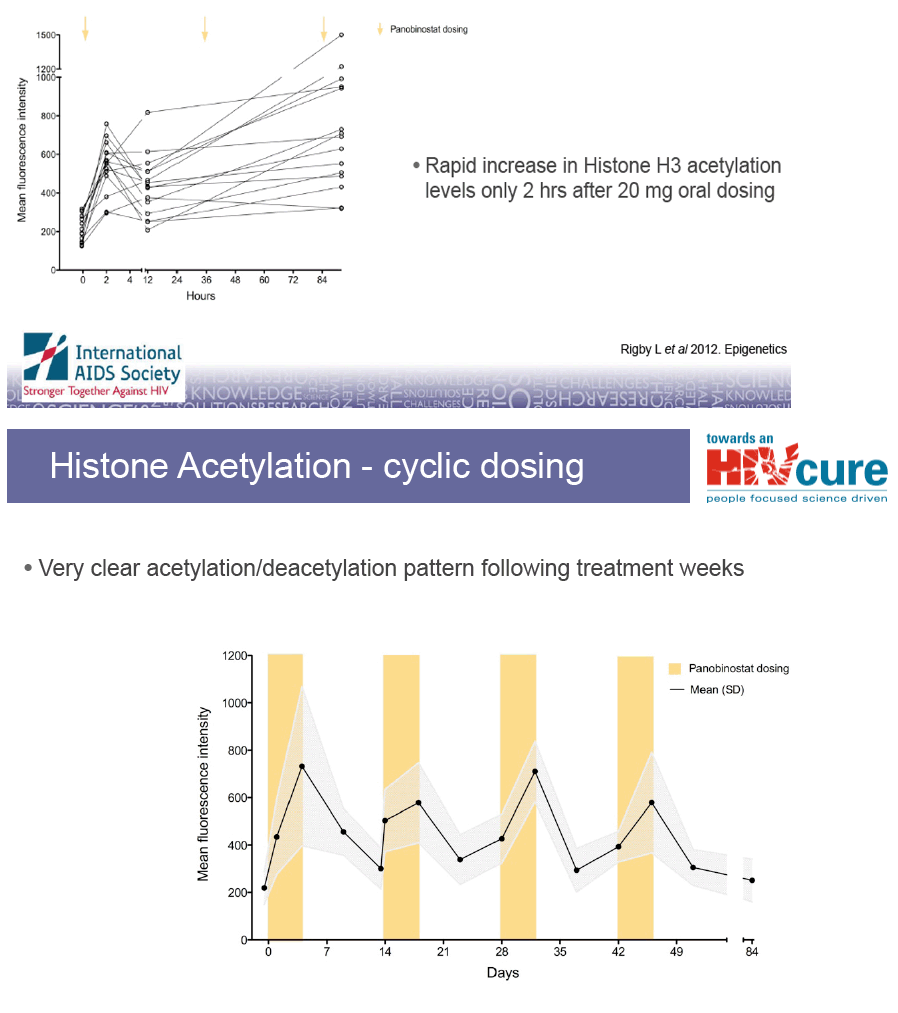
Could a cyclic dosing pattern lead to more efficient viral "kick" and reduce
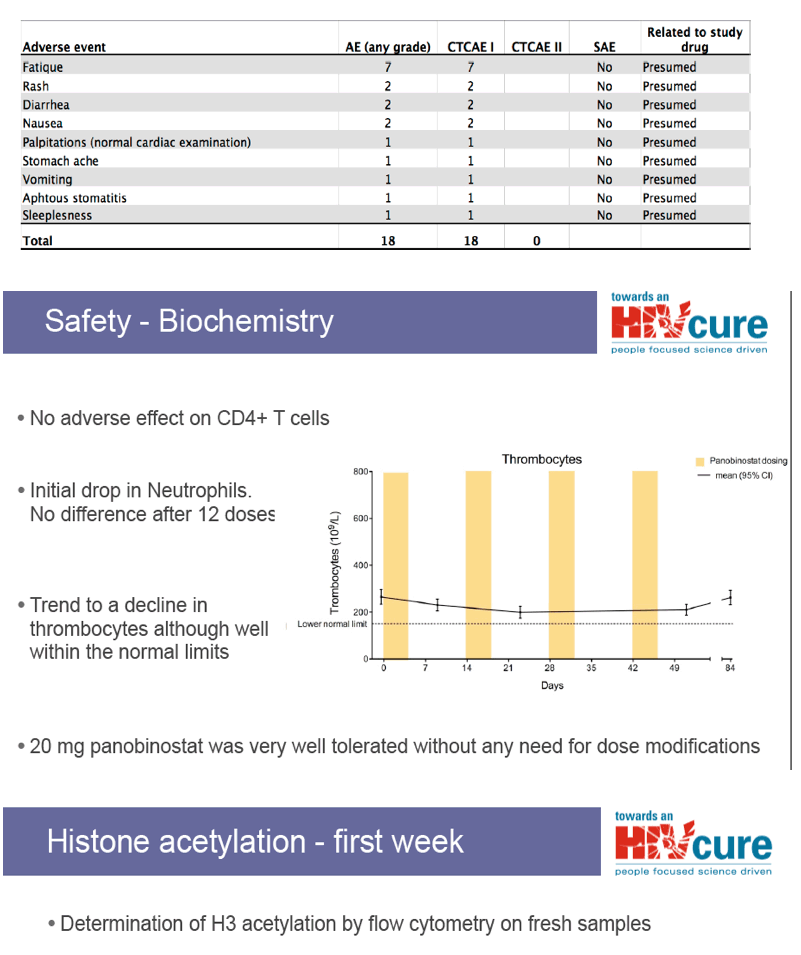
side effects especially thrombocytopenia?


|
| |
|
 |
 |
|
|