 |
 |
 |
| |
Long term therapeutic success of etravirine in switch and naive patients
|
| |
| |
Prolonged Response to Etravirine Regimens After Switch in Large Cohort Study
7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July
3, 2013, Kuala Lumpur
Link to webcast: http://pag.ias2013.org/flash.aspx?pid=246
Mark Mascolini
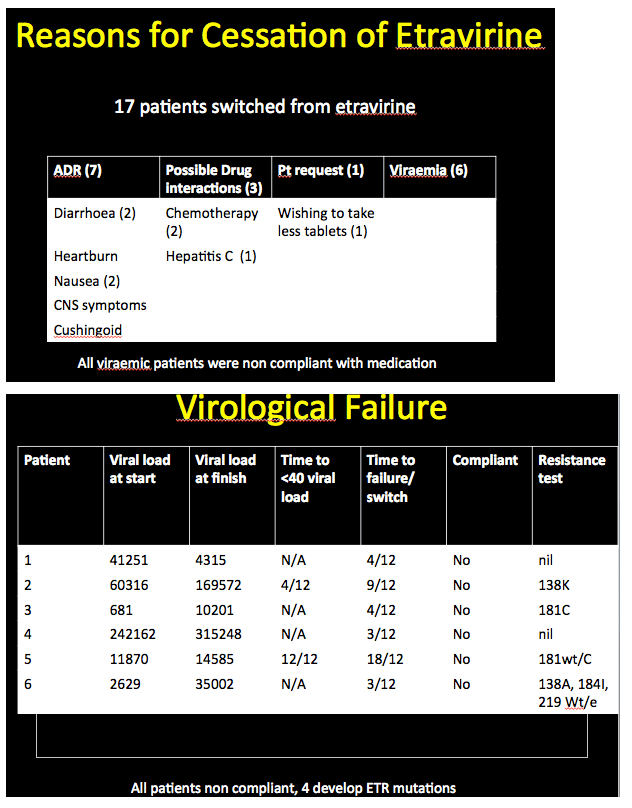
Good virologic responses to regimens built on the nonnucleoside etravirine proved frequent and durable in a 389-person retrospective case review from London's Chelsea and Westminster Hospital [1]. Among people who stopped etravirine because of virologic failure, all had evidence of poor adherence.
Etravirine, a second-generation nonnucleoside, is licensed in the United States for antiretroviral-experienced people at least 6 years old with a detectable viral load and evidence of HIV resistant to a nonnucleoside and other antiretrovirals [2]. Chelsea and Westminster clinicians mounted this case review to assess the efficacy and safety of etravirine in people with and without a detectable viral load when they started the drug. The researchers focused on all patients starting etravirine with two nucleosides at some point between August 2008 and December 2012.
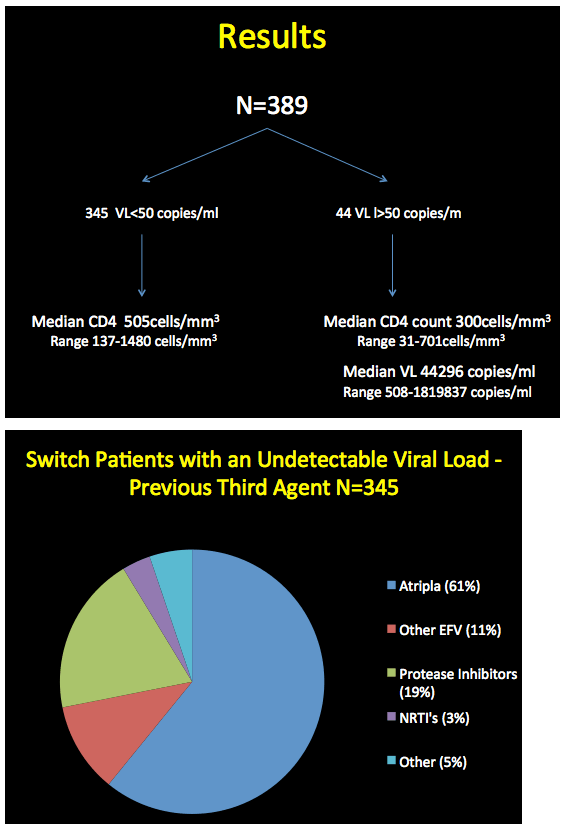
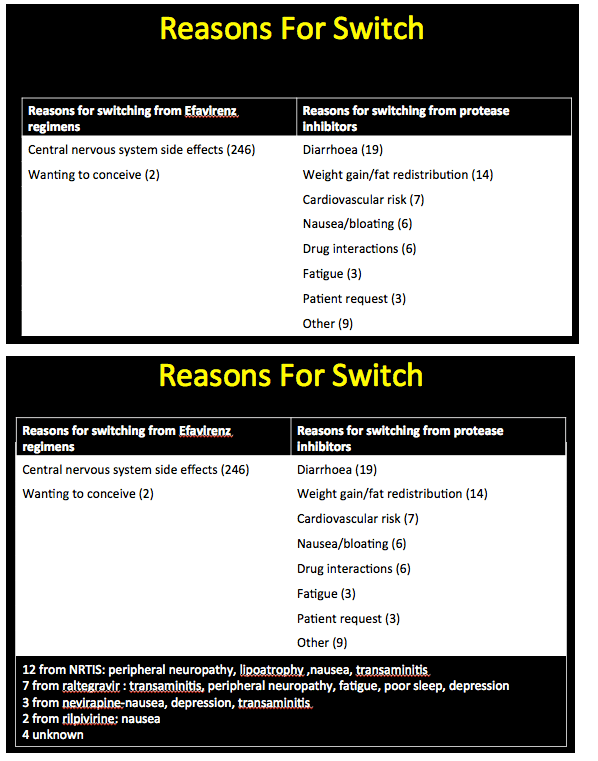
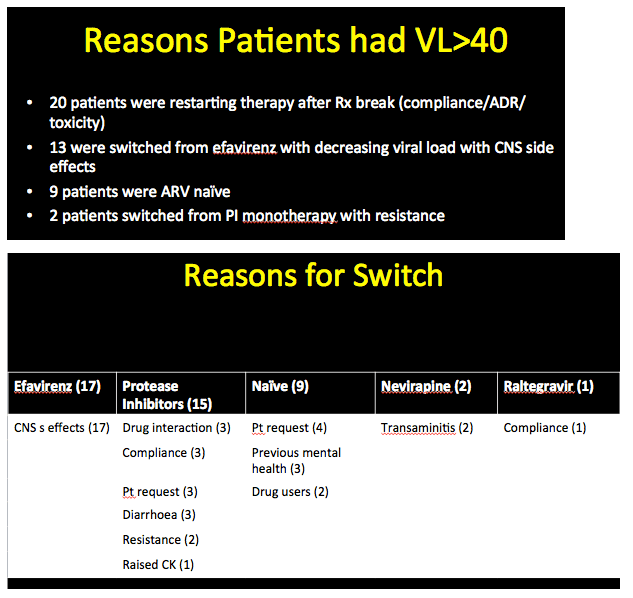
The analysis included 389 people, 345 who started etravirine with a viral load below 40 copies and 44 who started with a load above 40 copies. Median CD4 count stood at 505 in the sub-40-copy group (range 137 to 1480) and at 300 in the over-40-copy group (range 31 to 701). Among people with a detectable viral load, median load stood at 44,296 copies (range 508 to 1.8 million). Among the 345 people switching to etravirine with an undetectable viral load, 61% were taking Atripla, 11% another efavirenz regimen, and 19% protease inhibitors (PIs). Among the 248 switching from efavirenz, 246 did so because of neurologic side effects and 2 because they were planning to have a child. Among people switching from a PI, the most frequent reasons were diarrhea, weight gain, and abnormal fat distribution.
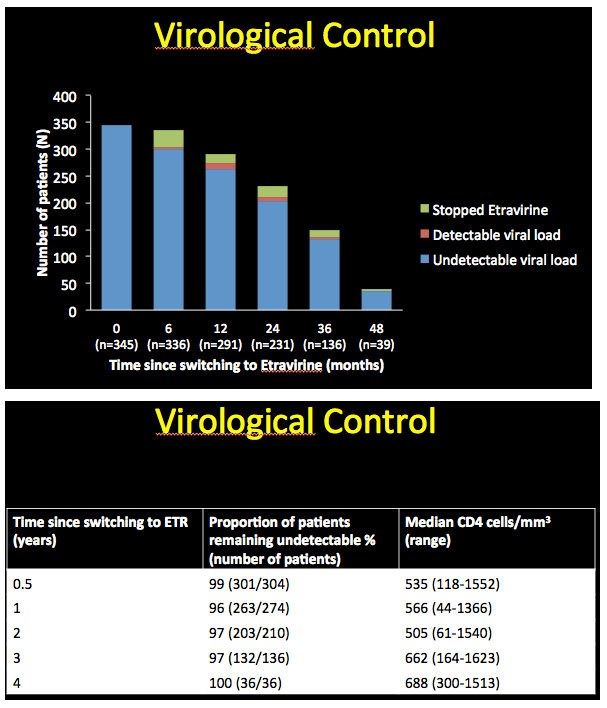
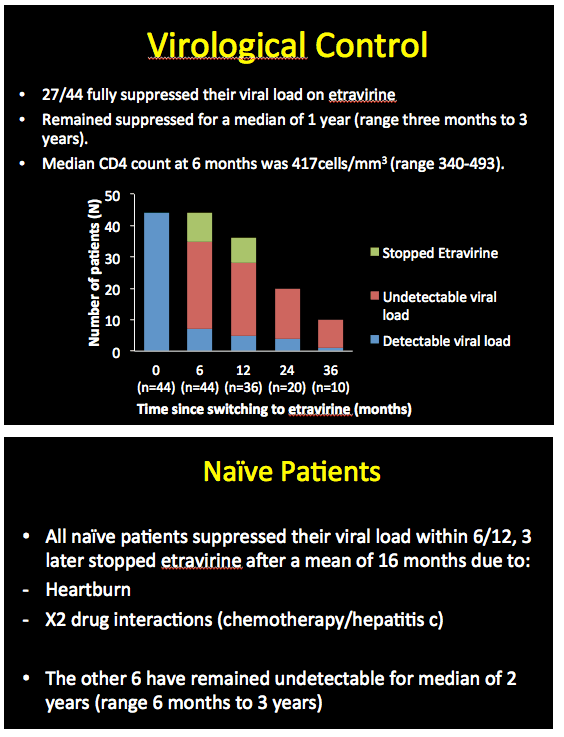
Of the 345 people who started etravirine with an undetectable viral load, proportions remaining undetectable were 99% at 6 months, 96% at 1 year, 97% at 2 years, 97% at 3 years, and 100% at 4 years. Median CD4 counts at those five points were 535, 566, 505, 662, and 688.
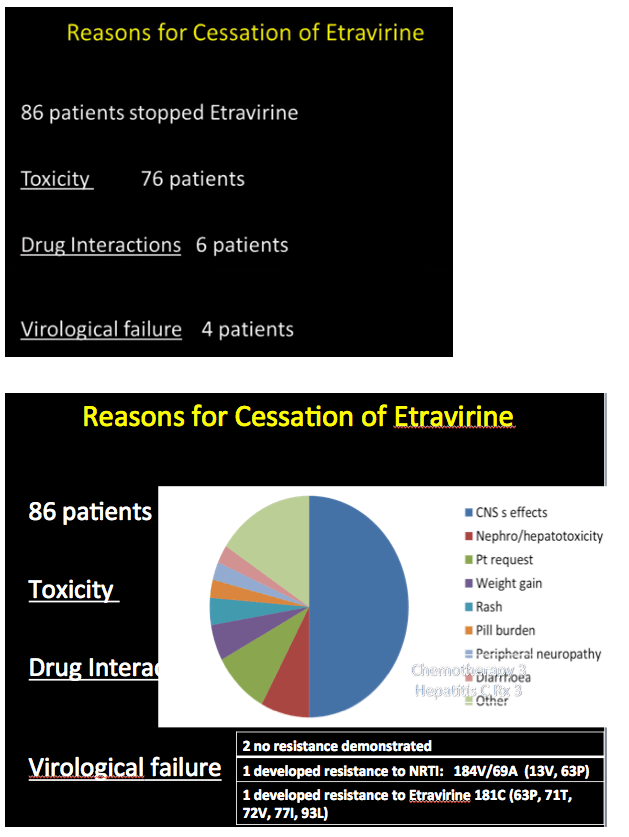
Among 86 of these 345 people (25%) who stopped etravirine during follow-up, 63 had taken efavirenz earlier. Thirty-one of these 63 (49%) stopped etravirine because central nervous system problems attributed to efavirenz persisted after the switch. New side effects included kidney or liver toxicity, weight gain, rash, peripheral neuropathy, and diarrhea. Only 3 people (3.5% of 86) stopped etravirine because of virologic failure. Two of these people had no evidence of new resistance mutations at failure; in the third the etravirine-related Y181C mutation emerged. The investigators noted that all 3 of these people with virologic failure adhered poorly to their regimen.
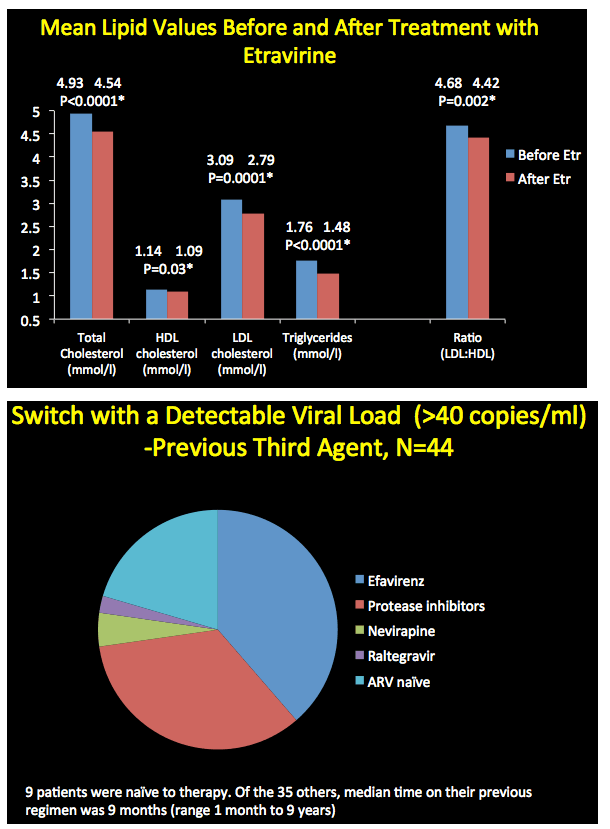
After patients started etravirine, total cholesterol, "bad" low-density lipoprotein (LDL) cholesterol, and triglycerides all fell significantly. "Good" high-density lipoprotein (HDL) cholesterol also fell significantly. The LDL/HDL cholesterol ratio improved slightly but significantly after the switch from 4.68 to 4.42 (P = 0.002).
Of the 44 people who started etravirine with a viral load above 40 copies, only 9 (20%) began etravirine as part of their first regimen. The other 35 had taken their previous regimen for a median 9 months (range 1 month to 9 years). Almost all of these switchers were taking either efavirenz or a protease inhibitor (including 2 on PI monotherapy with resistant virus). Twenty people were restarting therapy after a treatment break caused by poor adherence, a drug interaction, or toxicity. Thirteen people switched from efavirenz with a falling viral load but central nervous system side effects.
Among these 44 people who started etravirine with a detectable viral load, 27 (61%) attained an undetectable load during 36 months of follow-up. These people maintained virologic suppression for a median 1 year (range 3 months to 3 years). Among the 10 people who continued etravirine for 36 months, all reached and maintained an undetectable viral load. Among the 9 people who started etravirine as part of their first regimen, all reached a viral load below 40 copies within 6 months. Three of these 9 stopped etravirine after an average 16 months because of drug interactions in 2 and heartburn in 1. The other 6 all maintained an undetectable viral load for 6 months to 3 years (median 2 years).
Among the 44 people who started etravirine with a detectable viral load, 6 (14%) had virologic failure. Four of these 6 never reached an undetectable load with etravirine and all 6 adhered poorly to their regimen. Two of these 6 had no detectable resistance mutations at virologic failure.
The Chelsea and Westminster team proposed that "etravirine is an alternative switch option in individuals with an undetectable viral load and intolerant of their current third agent." They noted that only 1 person switching to etravirine with an undetectable viral load had a new etravirine resistance mutation through 803 patient-years of follow-up. The researchers stressed the need for further study of etravirine as a first-line antiretroviral.
References
1. Bull L, Bower M, Nelson M. Long term therapeutic success of etravirine in switch and naive patients. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur. Abstract WEAB0105.
2. AIDSinfo Drug Database. Etravirine. http://aidsinfo.nih.gov/drugs/398/etravirine/0/professional

|
| |
|
 |
 |
|
|