 |
 |
 |
| |
High Transplacental Transfer of Atazanavir But No Hyperbilirubinemia in Neonates
|
| |
| |
Download the PDF
7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur
Mark Mascolini
Transplacental passage of atazanavir yielded cord blood drug levels usually above the minimum effective concentration in a study of 15 mother-infant pairs [1]. Although none of the newborns had hyperbilirubinemia, a common finding in adults taking atazanavir, the Irish/UK investigators called for further study of the effects of fetal and neonatal atazanavir.
Transplacental transfer of antiretrovirals is a well-established phenomenon, but research is only starting to describe the extent of this transfer and its effects on neonates. Researchers in Dublin and colleagues at the University of Liverpool measured atazanavir and bilirubin levels in cord blood of 15 mother-infant pairs and in maternal blood at delivery. They also measured bilirubin in infants in the first 24 hours of life.
Six women started atazanavir before conception and 9 began the protease inhibitor between 19 and 33 weeks gestation (median 15 weeks). All women took the standard atazanavir/ritonavir dose of 300/100 mg once daily. Three women were antiretroviral naive when they started atazanavir, and 13 had a viral load below 50 copies at delivery. Viral loads in the 2 women with detectable viremia were 108 and 633 copies. Birth weight averaged 3.3 kg (range 2.0 to 4.7 kg). Two babies, twins, were born at 35 weeks gestation and the others were born after 37 weeks.
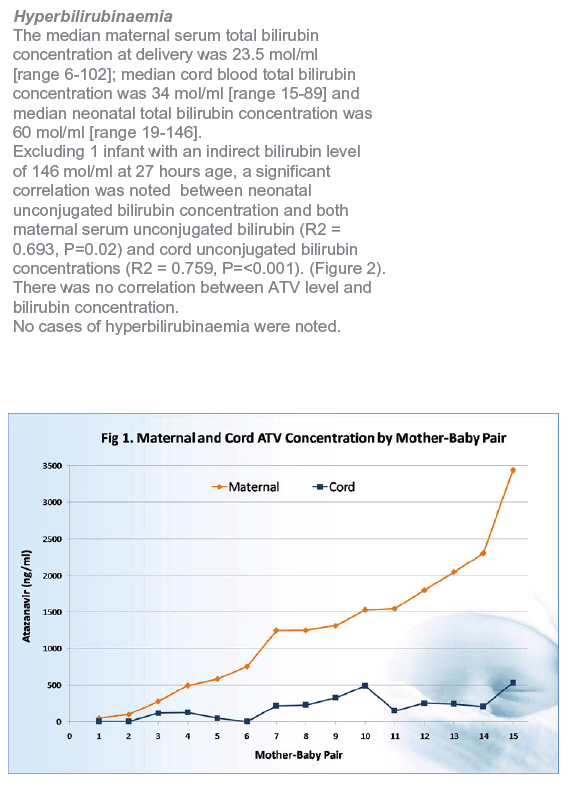
In 15 mother-infant pairs with available maternal and cord blood atazanavir concentrations, median maternal concentration was 1250 ng/mL (range less than 48 to 3441 ng/mL). Maternal concentration lay above the minimum effective concentration of 150 ng/mL in 13 of 15 samples. Twelve of 15 cord samples had detectable atazanavir concentrations at a median of 223 ng/mL (range less than 48 to 531 ng/mL). Atazanavir levels lay above the minimum effective concentration in 8 of 12 cord blood samples and 3 samples had borderline concentrations.
Linear regression analysis identified a significant correlation between maternal blood and cord blood atazanavir concentrations (R2 = 0.632, P < 0.001). Average ratio of maternal-to-cord atazanavir concentration was 0.14 (95% confidence interval [CI] 0.08 to 0.20).
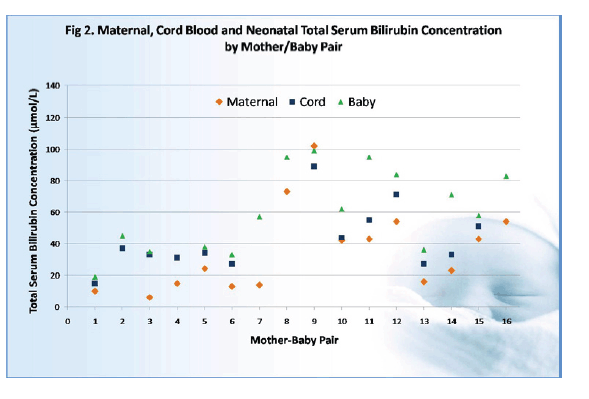
At delivery median maternal total bilirubin lay at 23.5 mol/mL (range 6 to 102 mol/mL), while median cord blood total bilirubin measured 34 mol/mL (range 15 to 89 mol/mL) and median neonatal total bilirubin 60 mol/mL (range 19 to 146 mol/mL).
When the researchers eliminated 1 infant with an indirect bilirubin concentration of 146 mol/mL 27 hours after delivery, they found a significant correlation between neonatal unconjugated bilirubin and (1) maternal unconjugated bilirubin (R2 = 0.693, P = 0.02) and (2) cord unconjugated bilirubin (R2 = 0.759, P
The researchers proposed that "transplacental transfer of atazanavir may offer additional protection to the neonate against mother-to-child transmission of HIV, with therapeutic levels observed in the majority of cord blood samples here." Although they found no cases of hyperbilirubinemia in this small study, they called for "further larger scale studies into the effects of atazanavir on the fetus and neonate."
Reference
1. Lambert J, Jackson V, Lawless M, et al. Atazanavir in pregnancy: transplacental transfer and neonatal hyperbilirubinaemia. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur. Abstract MOPE037. http://pag.ias2013.org/EPosterHandler.axd?aid=2331

|
| |
|
 |
 |
|
|