 |
 |
 |
| |
"The Clinical Role and Cost-effectiveness of Long-acting Antiretroviral Formulations" - Long-Acting ARV Formulations Could Improve Survival in People With Poor Adherence
|
| |
| |
(full slide presentation below)
IDWeek, October 2-6, 2013, San Francisco
Mark Mascolini
Long-acting antiretroviral (ARV) formulations, now in clinical development, could prolong survival in people with HIV, according to results of a modeling study [1]. But the benefit may hold true only in people with barriers to good adherence. Although long-acting antiretrovirals may not be cheap, the researchers determined they could be "a good value" when used mainly for poorly adherent patients.
Monthly or even quarterly injected antiretrovirals now being developed [2-4] would be a boon to anyone with HIV infection, but especially for those who have a hard time taking drugs every day. Researchers in the Massachusetts General Hospital modeling group and colleagues at other institutions conducted this analysis to estimate the impact of long-acting antiretroviral therapy (LA-ART) on survival and to calculate its lifetime cost and cost-effectiveness.
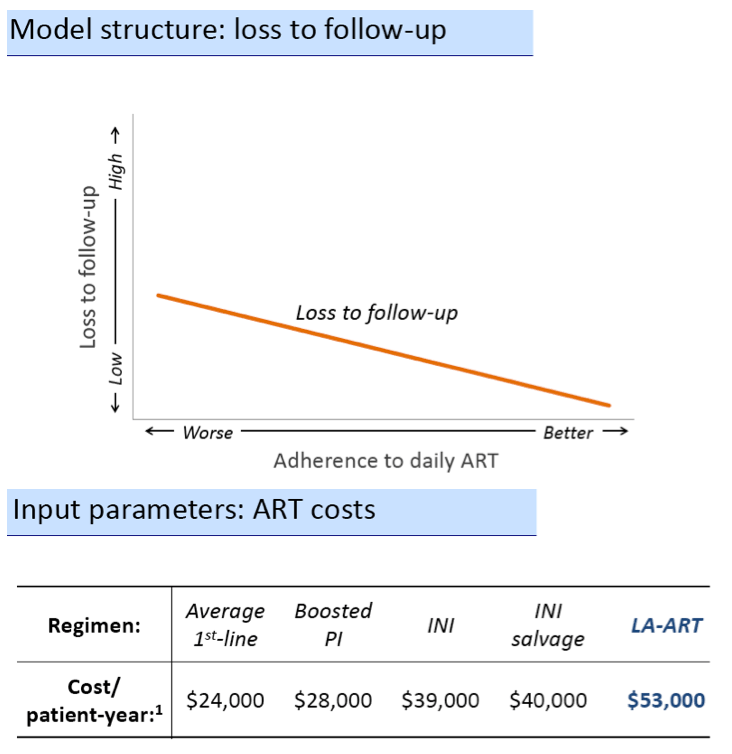
The Massachusetts General team used a simulation model of HIV disease progression that takes into account a person's CD4 count, viral load, antiretroviral use, and retention in care over a lifetime (the CEPAC-US model). The simulation began with (1) antiretroviral-naive people starting their first regimen with daily oral antiretrovirals. The investigators compared outcomes in this group with results in three other groups: (2) people with multiple virologic failures starting LA-ART, (3) people using LA-ART as second-line therapy after first-line failure, and (4) antiretroviral-naive people beginning treatment with LA-ART.
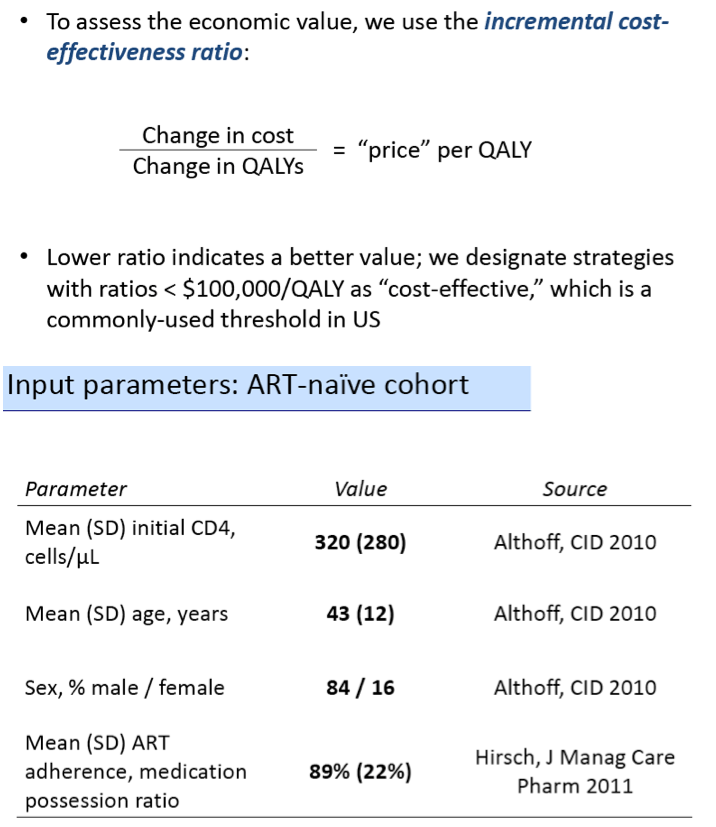
The modeling team classified strategies with incremental cost-effectiveness ratios below $100,000 per quality-adjusted life year (QALY) as cost-effective. The antiretroviral-naive cohort modeled had an average CD4 count of 320, an average age of 43, an average adherence (measured as medication possession ratio) of 89%, and a male/female ratio of 84/16. The model used an average first-line regimen cost of $24,000 per patient per year, $28,000 for a boosted protease inhibitor regimen, $39,000 for an integrase inhibitor regimen, $40,000 for an integrase inhibitor salvage regimen, and $53,000 for LA-ART.
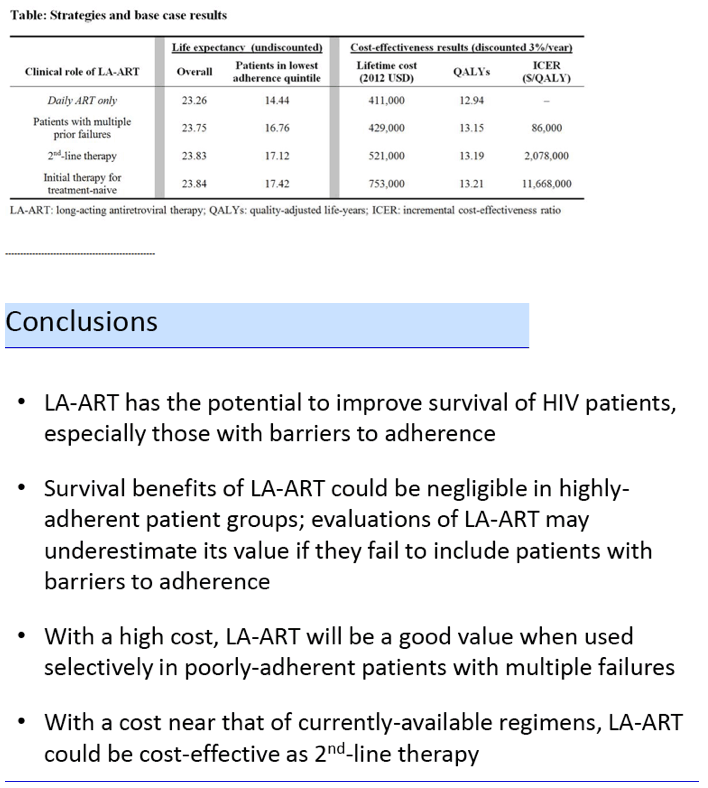
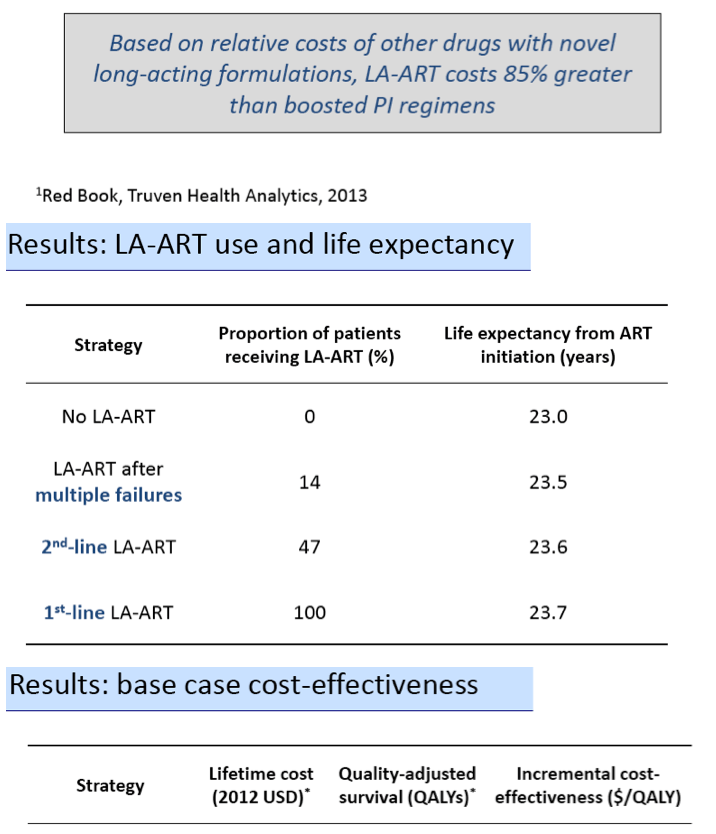
The model determined that life expectancy would be lowest with daily oral antiretrovirals and progressively longer with LA-ART for multiple failures, second-line LA-ART, and first-line LA-ART:
Life expectancy from the start of ART:
1. First-line daily oral ART (0% on LA-ART): 23.0 years
2. LA-ART after multiple failures (14% on LA-ART): 23.5 years
3. Second-line LA-ART (47% on LA-ART): 23.6 years
4. First-line LA-ART (100% on LA-ART): 23.7 years
Lifetime cost, QALYs, and cost per QALY also rose with each successive strategy:
Lifetime cost, QALYs, and cost per QALY:
1. First-line daily oral ART: $400,000, 12.67 QALYs
2. LA-ART after multiple failures: $420,000, 12.88 QALYs, $90,000/QALY
3. Second-line LA-ART: $490,000, 12.96 QALYs, $980,000/QALY
4. First-line LA-ART: $670,000, 12.99 QALYs, $6,190,000/QALY
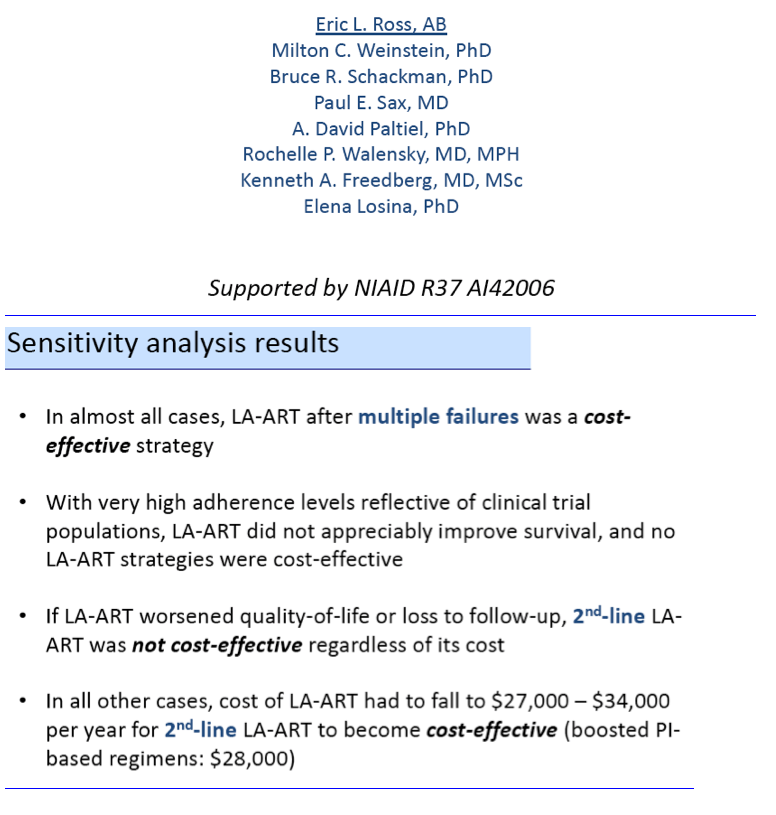
Thus only LA-ART after multiple failures met the definition of cost-effectiveness (below $100,000).
The researchers cautioned that this is "an exploratory analysis" because no one knows what the actual cost, efficacy, and tolerability of LA-ART will be. They noted that their cost-effectiveness analysis does not factor in one likely impact that would probably enhance the value of LA-ART: lower HIV transmission by people on LA-ART.
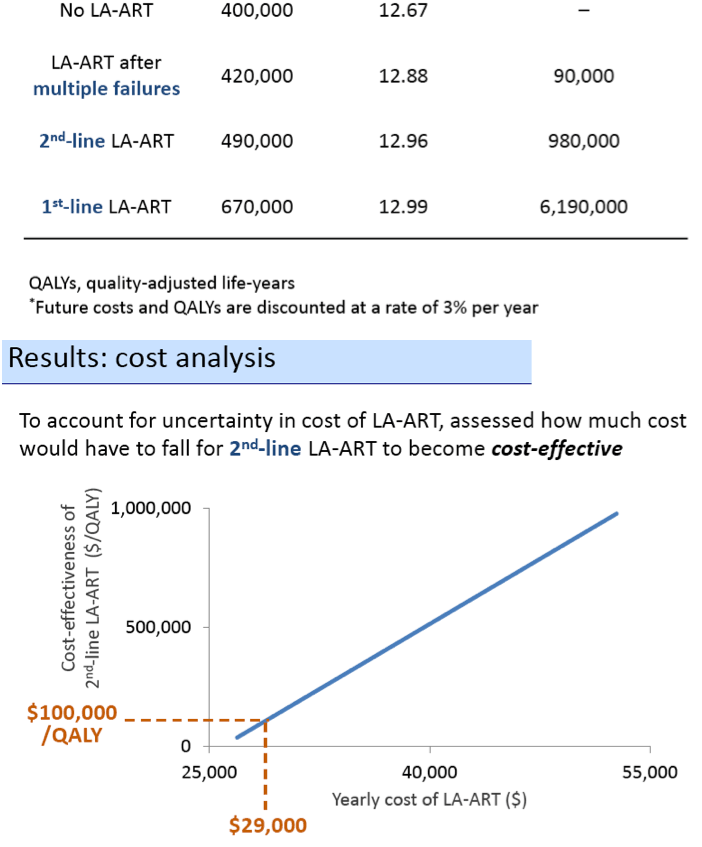
The investigators concluded that LA-ART may improve survival of HIV-positive people when compared with today's standard daily oral regimens. But if these modeling projections are accurate, people already highly adherent to daily oral ART would probably derive little survival benefit from LA-ART. Because of its projected high cost, the investigators cautioned, LA-ART will be "a good value" only when used selectively for poorly adherent people with a record of multiple antiretroviral failures (cohort 2). But if LA-ART costs only a little more than current oral regimens, it could also be cost-effective as second-line therapy (cohort 3).
References
1. Ross E, Weinstein M, Schackman B, et al. The clinical role and cost-effectiveness of long-acting antiretroviral formulations. IDWeek 2013. October 2-6, 2013. San Francisco. Abstract 78.
2. Mascolini M. High levels of two antiretrovirals with monthly or quarterly injections in healthy volunteers: GSK744 LAP + TMC278 LA. NATAP. IAS 2013. http://www.natap.org/2013/IAS/IAS_63.htm
3. Mascolini M. Good safety profile with long-acting integrase inhibitor, GSK744. 53rd ICAAC, 2013. http://www.natap.org/2013/ICAAC/ICAAC_07.htm
4. Levin J. Antiviral characteristics of S/GSK1265744, an HIV integrase inhibitor dosed by oral or long-acting parenteral injection: once monthly or 3 months dosing/PrEP/HAART. 52nd ICAAC, 2012. http://www.natap.org/2012/ICAAC/ICAAC_12.htm
PROGRAM ABSTRACT
Background:
Long-acting antiretroviral formulations (LA-ART), currently in development, aim to achieve monthly or quarterly ART dosing; this could improve health benefits of ART for HIV-infected individuals who have difficulty maintaining daily adherence. We sought to identify the clinical and economic circumstances under which differing clinical roles of LA-ART might be cost-effective in the US.
Methods:
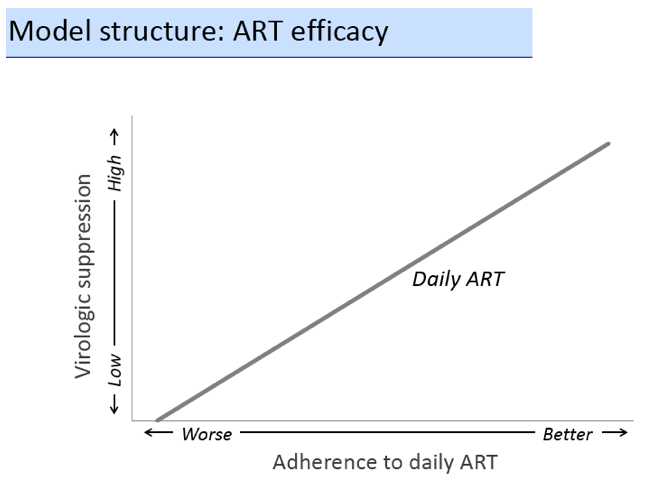
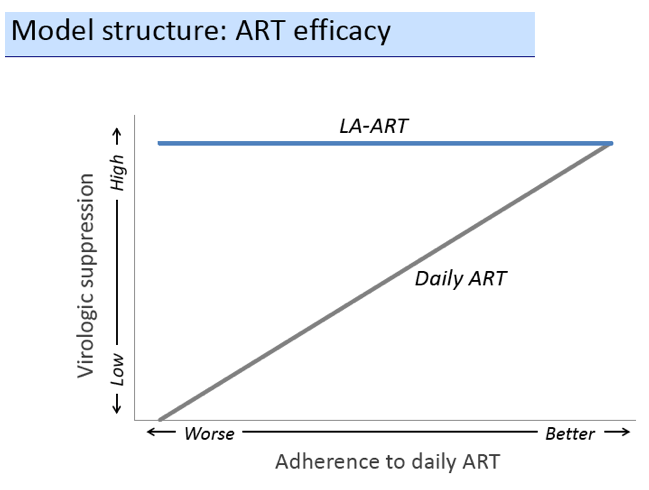
We used a microsimulation model of HIV disease progression (CEPAC-US) to project the impact of 3 potential roles of LA-ART (compared to daily ART only): 1) initial therapy for all ART-na´ve patients, 2) 2nd-line therapy for those failing 1st-line, and 3) use for patients with multiple prior failures on NNRTI- and PI-based regimens. Model outcomes include quality-adjusted life-years (QALYs), lifetime cost, and incremental cost-effectiveness ratio (ICER); strategies with ICER < $100,000/QALY are designated "cost-effective". We simulate a cohort with mean adherence (medication possession ratio) of 89% (SD = 22%). Depending on adherence, HIV RNA < 400c/mL at 48 weeks on daily ART ranges from 0 to 91%, and loss to follow-up ranges from 41 to 4/100PY. We assume LA-ART's efficacy is 91% regardless of adherence to daily ART, and that LA-ART costs $60,000/patient-year (vs. $28,000 for daily PI-based regimens). In sensitivity analysis, we vary LA-ART's cost, efficacy, and quality of life (QOL) impact (due to benefits of reduced pill burden or detrimental side effects).
Results:
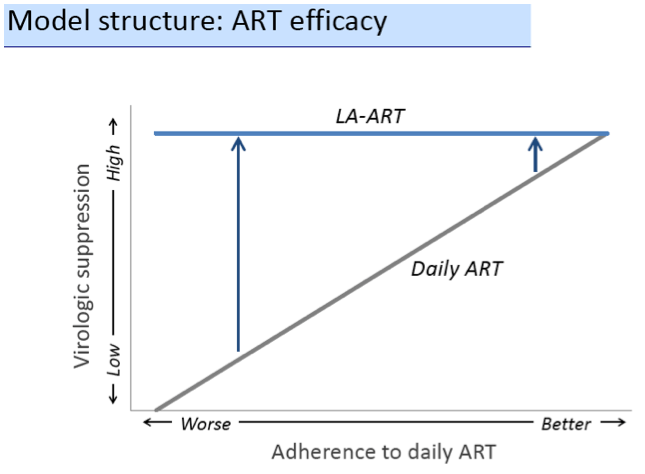
In the base case, LA-ART increases overall life expectancy (LE) compared to daily ART by 0.5-0.6 years, and LE of patients with the lowest adherence by 2.3-3.0 years, depending on clinical role; only LA-ART for patients with multiple failures is cost-effective ($86,000/QALY, Table). With a cost of $30,000/year and a favorable QOL impact, 2nd-line LA-ART is cost-effective ($94,000/QALY); varying efficacy of LA-ART has minimal impact on cost-effectiveness results.
Conclusion:
LA-ART could improve survival of US HIV patients, especially those with barriers to adherence and poor outcomes on daily ART. With a high cost, it will be a good value for use in patients with multiple prior failures; a cost close to current regimens combined with demonstrable QOL benefit would support broader use.
--------------------------------------------------


|
| |
|
 |
 |
|
|