 |
 |
 |
| |
Clinical Pharmacology at the
14th Workshop on Clinical Pharmacology of HIV Therapy and HCV
|
| |
| |
Courtney V. Fletcher, Pharm.D.
Dean and Professor
College of Pharmacy
University of Nebraska Medical Center
986000 Nebraska Medical Center
Omaha, NE 68198
The 14th International Workshop on Clinical Pharmacology of HIV Therapy was held in Amsterdam, The Netherlands from April 22-24, 2013. In this report I will highlight abstracts focused on pharmacologic issues I think are of broad interest or might benefit from some expert clarification. I will discuss abstracts in three broad categories: compartmental penetration of ARVs and PrEP; pharmacogenetics and drug-drug interactions; and clinical pharmacology of HCV therapy and drug interactions.
Topics
- ARV concentrations in the CSF are associated with CSF viral load levels below 50 copies/mL.
- The effect of patient characteristics on long-acting rilpivirine (RPV-LA) PK
- Plasma and tissue PK of GSK-744
- Pharmacogenetics and Drug-Drug Interactions: rifampin & efavirenz
- Hepatitis C Coinfection: BOC (Boceprevir) with ATV/RTV (Reyataz) or RAL
(raltegravir) in HIV and HCV co-infected persons; The effect of BOC and TVR (telaprevir) on maraviroc (MVC); The effect of BOC and TVR on dolutegravir (DTG) PK
Abbreviations
%CV, percent coefficient of variation
ABC, abacavir
ACTG, adult AIDS clinical trials group
APV, amprenavir
ARV, antiretroviral drug
ART, antiretroviral drug therapy
AUC, area under the concentration-time curve
ATV, atazanavir
BOC, boceprevir
C12, drug concentration at 12 hours post dose
Cmax, maximum drug concentration
Cmin, minimum drug concentration
CVC, cenicriviroc
CNS, central nervous system
COBI, cobicistat
CSF, cerebrospinal fluid
CVF, Cervicovaginal fluid
Ctrough, concentration immediately before the next dose
CYP, cytochrome P450 drug metabolizing enzymes
DBS, dried blood spot
DHHS, Department of Health and Human Services
DSMB, data safety monitoring board
DTG, dolutegravir
DRV, darunavir
ddI, didanosine
EFV, efavirenz
EVG, elvitegravir
FDV, faldaprevir
FTC, emtricitabine
ETR, etravirine
fAPV, fosamprenavir
GMR, geometric means ratio
HAND, HIV-associated neurocognitive disorders
HDAc, histone deacetylase
ICxx, concentration of drug required to inhibit viral replication in vitro by xx%, where xx may be 50%, 90%, 95%.
IDV, indinavir
IM, intramuscular
IQ, inhibitory quotient
IVR, intra-vaginal ring
3TC, lamivudine
LDV, ledipasvir
LPV, lopinavir
MVC, maraviroc
NVP, nevirapine
NRTI, nucleoside reverse transcriptase inhibitor
NNRTI, non-nucleoside reverse transcriptase inhibitor
PACTG, pediatric AIDS clinical trials group
PBMCs, peripheral blood mononuclear cells
PD, pharmacodynamic
PG, pharmacogenetics/pharmacogenomics
PK, pharmacokinetic
PI, inhibitor of HIV protease
PrEP, pre-exposure prophylaxis
r or RTV, ritonavir
RAL, raltegravir
RBT, rifabutin
RBV, ribavirin
RPT, rifapentine
RIF, rifampin
RPV, rilpivirine
SQV, saquinavir
SC, subcutaneous
SOF, sofosbuvir
TAF, tenofovir alafenamide
TDF, tenofovir disoproxil fumarate
TFV, tenofovir
TVR, telaprevir
TDM, therapeutic drug monitoring
TPV, tipranavir
TB, tuberculosis
ZDV, zidovudine
I. Compartment Penetration of ARVs and PrEP
1. ARV concentrations in the CSF are associated with CSF viral load levels below 50 copies/mL.
Calcagno and colleagues from Torino evaluated ARV concentrations, the extent to which the concentrations were above the IC50 or IC95 and CSF viral loads (pharmacodynamic effect) in 113 patients. 150 CSF samples were measured for ARV concentrations: 90 (60%) were above the IC95; 52 (35%) were between the IC95 and IC50; and 8 (5%) were below the IC50. The drugs with the highest ratio of concentration-to-IC95 were DRV (11.3 for once daily) and EFV (6.4). The drugs with the ratios less than 1 (therefore, CSF concentrations are less than the IC95) were: RAL (0.6), MVC (0.4), NVP (0.3) and unboosted ATV (0.3). Overall, 62% of patients had HIV RNA in plasma that was < 50 copies/mL, and 53% of patients had CSF viral load < 50 copies/mL. The ratio of ARV concentration in the CSF-to-IC95 was significantly associated with a higher probability of a CSF viral load < 50 copies/mL (odds ratio 2.2), whereas the CSF-to-IC50 ratio was not.
The ratio of the concentration of an ARV to a measure of in vitro susceptibility (such as IC50 or IC95) provides an understanding of how much drug you have (the concentration) to how much drug you need (the ICxx). The higher the ratio, the greater the effect (up to the maximum extent possible) and the greater the tolerance for interpatient variability, drug-drug interactions, etc.
Conventionally, the IC50 is commonly used, though there is little evidence this is the best measure of in vitro susceptibility for predicting the in vivo pharmacodynamic effect. These data provide evidence that for the CSF, the degree to which ARV concentrations are above the IC95 provides a higher likelihood of achieving an undetectable CSF viral load. These data should drive an examination of the best in vitro measure for predicting effect at a given ARV concentration, and may warrant further evaluation of CSF-targeted ARV therapy.
2. The effect of patient characteristics on long-acting rilpivirine (RPV-LA)
PK. SSAT040 is a study of RPV-LA in female and male healthy volunteers. Several abstracts have been presented on this study. In this analysis, the influence of participants' characteristics (gender, race, age, BMI [body mass index]) on the PK of RPV was evaluated. 66 persons (60 female) were enrolled. Females received a single IM injection of 300, 600 or 1200 mg. Males received a single injection of 600 mg. Plasma PK was evaluated as was cervicovaginal fluid (CVF) and vaginal tissue (VT) in females and rectal fluid (RF) and tissue (RT) in males. The Cmax of RPV, which reflects absorption of the IM dose, was significantly higher (and independently associated) in males and in persons with a lower BMI. The 84-day AUC of RPV in plasma was associated with gender, and like Cmax was higher in males than in females. RPV concentrations in CVF (viewed as AUC) were associated with concentrations in plasma and higher in females ≥ 40 years and in those with a BMI < 25. Vaginal tissue concentrations were also higher in females with a BMI < 25. These results are informative as to patient characteristics that significantly influence drug concentrations. They provide an argument that "one dose is not likely to fit all".
3. Plasma and tissue PK of GSK-744. GSK1265744 (or GSK744) is a potent
integrase inhibitor. When administered as a single intramuscular injection to healthy volunteers in a long-acting nanosuspension formulation, the drug had a half-life of 21 to 50 days. Abstract O_02 evaluated the plasma and tissue PK of GSK744 in healthy male and female volunteers. 16 male and female volunteers received 400 mg IM either a single IM injection or split into two, 200 mg IM injections. Approximately two-fold higher peak concentrations were achieved with the split dose. Over the 36 weeks that plasma concentrations were measured, there was no difference in the overall AUC between the single and split doses. The median ratio of GSK744 concentrations in cervicovaginal tissue to plasma ranged from 16% to 28%. The median ratio of rectal tissue to plasma (obtained only from male participants) was ≤ 8%.
The 400 mg dose of GSK744 used in this study was, as expected by the investigators, a subtherapeutic dose. The higher concentrations achieved by splitting the dose suggests this may be a strategy to rapidly achieve therapeutic concentrations. The association between higher tissue concentrations with higher plasma concentrations suggests the low tissue concentrations may be improved with a higher dose. At CROI-2013 (abstract 24LB), data were presented on the efficacy of GSK744 for PrEP in 8 macaques that received intramuscular doses of GSK744 at two time points 4 weeks apart. Dosing started 1 week before intrarectal exposures to SHIV, which were given weekly for up to 8 exposures. All eight of the control macaques receiving placebo became infected with SHIV. None of the 8 treated macaques had detectable virus 3 weeks after the final viral challenge. Collectively, the human PK data and the macaque efficacy data suggest real promise for GSK744 as an agent for PrEP. However, there is a need for PK studies that demonstrate a large enough dose can be administered to achieve therapeutic plasma and tissue concentrations, prior to any efficacy studies in humans.
CROI: PrEP GSK744 Integrase Administered Monthly Perhaps Quarterly Prevents HIV-Infection in Monkeys - (03/07/13)
CROI: Long-Acting Integrase Inhibitor Shields Macaques From Anal Simian HIV - written by Mark Mascolini - (03/04/13)
ICAAC: Lack of Pharmacokinetic (PK) Interaction Between Rilpivirine and the Integrase Inhibitors Dolutegravir and S/GSK1265744 - (09/12/12)
ICAAC: Antiviral Characteristics Of S/GSK1265744, An HIV Integrase Inhibitor (INI) Dosed By Oral Or Long-acting Parenteral Injection....once monthly or 3 months dosing/PrEP/HAART - (09/10/12)
ICAAC: Resistance Findings on Experimental S/GSK Long-Acting Integrase Inhibitor - written by Mark Mascolini - (09/12/12)
II. Pharmacogenetics and Drug-Drug Interactions
In December 2011, the FDA modified efavirenz (EFV) dosing in the label to recommend an increase in the dose of EFV to 800 mg once daily in individuals who are taking concomitant rifampin (RIF) and weigh more than 50 kg. There are conflicting data as to the effect of RIF coadministration on EFV PK, and a lack of worldwide consensus on this recommendation. Importantly, new data are providing further insights into some of the paradoxical findings (e.g. why EFV concentrations may be increased in some persons taking RIF [and isoniazid]). Three relevant and important abstracts were presented at this meeting.
Abstract O-04 investigated the influence of CYP2B6 genotype and rifampin on the PK of EFV in Ugandans. 263 HIV-infected Ugandans were recruited into the study. 106 were ART-naïve and were started on EFV/3TC/ZDV. 157 were HIV and TB coinfected: rifampin (RIF) based TB therapy (that also included isoniazid - see below) was started 2 weeks prior to an EFV-containing ART regimen. Plasma samples for EFV were collected periodically over almost 1 year of EFV dosing. Mean body weights were 55 kg in the HIV cohort and 50 kg in the HIV+TB cohort. In the first 56 days, the clearance of EFV was higher in the EFV and RIF cohort than in the EFV only. This most likely reflects the enzyme inducing effect of RIF. Interestingly, after day 56 through day 224, there was no difference in EFV clearance between the two cohorts. Overall, interpatient variability in EFV clearance (or concentrations) was better explained by CYP2B6 genotype (2B6 is the primary enzyme responsible for EFV metabolism) than whether EFV was co-administered with RIF (and the influence of a drug-drug interaction).
In Abstract O_05 the influence of CYP2B6 and NAT2 genotypes on EFV PK were investigated in 307 TB and HIV coinfected Cambodian persons. The TB regimen was RIF plus isoniazid (INH) for 6 months with ethambutol and pyrazinamide for the first 2 months of TB therapy. In the setting of TB therapy, this study investigated either early or late initiation of ART: EFV (600 mg/day) plus d4T and 3TC. EFV blood samples were obtained during TB and ART therapy and only on ART after discontinuation of TB therapy. The median body weight was 44 kg at entry and was 54 kg at week 50. As expected, the CYP2B6 genotype was the most significant influence on EFV clearance (CL/F): 2B6 "fast" metabolizers had a CL/F of 12.5 L/h; intermediate, 8.8 L/h; and slow, 2.5 L/h. EFV CL/F was also influenced by NAT2 genotype and INH concentrations. EFV CL/F was decreased between 10%-25% in INH slow metabolizers (about 40% of the study population), while it was increased 8%-24% in INH fast metabolizers. 99% of participants had virologic success, defined as HIV RNA < 240 copies/mL, at week 50.
Abstract O_12 described a clinical trial simulation of the effectiveness 4 different doses of EFV (800, 600, 400 and 300 mg once daily). This trial simulation included the effects of a variety of factors known to affect EFV PK: body weight, adherence, CYP2B6 genotype, and RIF. Adherence was modeled as either perfect (i.e., 100%), or as the expected population median of 80%.
Clinical trial simulations of 10,000 patients treated for 6 months were performed. With standard dose EFV at the 80% level of adherence, the simulations indicated a virologic failure rate between 10%-20% could be expected (based on the influence of adherence, weight and CYP2B6 genotype). The addition of RIF increased the estimated virologic failure rate with the standard EFV dose to between 15%-28%. This was a very rigorous and thorough simulation study. The value of these simulation studies is they allow exploration of "experiments" that won't or can't be conducted - in this case a study of EFV considering the combined effects of weight, CYP2B6 genotype, the presence or absence of RIF, and medication adherence in 10,000 patients.
The STRIDE study (ACTG 5221) was an open label, randomized study comparing early vs. late initiation of ARV therapy in HIV-infected persons receiving RIF-based TB therapy. A PK study of EFV concentrations in 543 participants has recently been published (see Luetkemeyer AF, et al. Clin Infect Dis, advanced publication April 19, 2013). Median body weight was 52.8 kg; 74% were Black; 71% of participants were from sub-Saharan Africa. Overall, there was no difference in the EFV trough concentration on-RIF or off-RIF: median, 1.96 μg/mL on-RIF vs. 1.80 off-RIF, p=0.067. EFV concentrations were significantly higher in Blacks on-RIF compared with off-RIF: 2.08 μg/mL vs. 1.75 μg/mL, p=0.005. There was a trend for lower EFV concentrations in White and Hispanic participants on-RIF compared with off-RIF. EFV concentrations were significantly lower in those who weighed ≥ 60kg vs. those < 60kg: 1.68 μg/mL vs. 2.02 μg/mL, p=0.021. Body weight of ≥ 60kg, however, was associated with more frequent HIV RNA < 400 copies/mL at week 48: ≥ 60kg, 81.9%; < 60kg, 73.8%; p=0.023. Participants who had any or all EFV trough concentrations < 1 μg/mL while receiving RIF had significantly lower odds of achieving virologic suppression (OR, 0.57 and 0.49, respectively). It is important to note that 805 of the 806 participants (99.9%) in the STRIDE study were receiving isoniazid (see comments related to the interaction of INH and EFV in abstract O_05 above). These authors of this PK study concluded "These data do not support weight-based dose increase of EFV during rifampin-based TB treatment." As written, I disagree. 71% of the study population was from sub-Saharan Africa and 74% were Black. This population simply does not represent the world's population of HIV and TB infected persons who may receive concomitant EFV, RIF and INH, and their genetic diversity with respect to CYP2B6 (for EFV) and NAT2 (for INH), which significantly influence the effect on EFV PK.
Let me offer a few overall observations about EFV dosing when given with RIF and INH, and then a recommendation.
· The PK of EFV are clearly dependent upon CYP2B6 genotype, body weight, time and the presence and concentrations of inhibitors or inducers of relevant drug metabolizing enzymes; other metabolic pathways such as CYP2A6 appear relevant at least in certain settings.
· The evaluation, mechanistic understanding and clinical management of the interaction between EFV and RIF, must take into account whether INH is given as well.
· The PK consequence of the competing drug-drug interactions among EFV, RIF and INH is significantly influenced by the patient's CYP2B6 and NAT2 genotypes; the contribution of other host genetic characteristics cannot be ruled out at this time.
My recommendation.
Presently, there is simply no way to predict the EFV concentration an individual patient will have based on any characteristic (e.g. body weight, gender, CYP2B6 genotype). In the presence of interacting drugs (such as RIF and INH), the uncertainty as to the concentration is enhanced further. The concentration of EFV has clinical significance, as shown in the STRIDE PK study and by other investigators, because the risk of virologic failure was increased in patients who had EFV trough concentrations < 1 μg/mL.
I have always taken a very conservative approach to therapeutic drug monitoring (TDM). This setting - the HIV and TB coinfected person who is receiving EFV, RIF and INH, I believe is a clinical scenario where the best management approach, to ensure the highest probability of virologic success with EFV, is to perform TDM for EFV. Otherwise, some patients will fail EFV therapy for preventable reasons! It is true there is not widespread availability of the analytical method to quantify EFV in plasma. However, this is a minor technical consideration that can be overcome if there is a will to do it. EFV plasma concentrations can accurately be measured by HPLC (no mass spectrometers are necessary) and the procedure is no more difficult than measuring phenytoin concentrations to appropriately manage patients who have seizure disorders that is widely available. Such an assay is quite inexpensive, and much, much less than the cost of virologic failure. In my opinion, the evidence is quite compelling that EFV TDM to manage these competing drug interactions is the best and most cost-effective approach.
III. Hepatitis C Coinfection
A theme at this meeting and at CROI this year was the progress made in the discovery, development and clinical pharmacology of drugs to treat hepatitis C (HCV). Here are three abstracts that provide information about drug-drug interactions between telaprevir (TVR) and boceprevir (BOC) and ARVs.
1. BOC with ATV/RTV or RAL in HIV and HCV co-infected persons.
Abstract O-15 is a very small study of the PK of ATV/RTV or RAL when given in combination with BOC. What makes this abstract interesting is it is the first, to my knowledge, to look at these interactions in HIV and HCV coinfected persons versus healthy volunteers. 12 patients completed this study, 7 on ATV/RTV and 5 on RAL (both given with other ARVs). PK sampling was performed during the 4-week lead in phase of HCV therapy with peg-IFN and ribavirin (off-BOC) and again after BOC was added (on-BOC). The table below shows the mean PK parameters and mean ratios on-BOC/off-BOC.
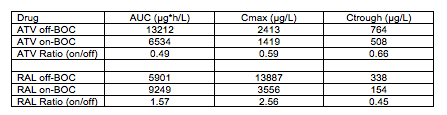
These data show that ATV concentrations are reduced in the presence of BOC, and are consistent with magnitude of reduction seen in healthy volunteers (see Hulskotte E et al. Clin Infect Dis 2013;56:718-26). Also consistent was that BOC concentrations were not changed to a clinically significant degree. With regard to coadministration, the FDA has recommended that BOC and ATV/RTV not be coadministered, while the European Medicines Agency (EMA) recommended that coadministration could be considered on a case-by-case basis. The basis for the EMA recommendation can be seen in these data. While ATV trough concentrations are reduced by approximately 40%, they are above those generally considered to be associated with therapeutic response (150 ng/mL). My preference would be to avoid this combination until more data are available. But, if clinicians really had no other options, then I think it could be considered with very close monitoring of the HIV virologic response.
As for combining BOC and RAL, these data also are generally consistent with those in healthy volunteers (see de Kanter C et al. Clin Infect Dis 2013;56:300-6). There was no effect on BOC concentrations. The RAL AUC and Cmax were not decreased. In these 5 HIV and HCV coinfected persons, the trough concentration of RAL was decreased by 50%; a decrease was observed in healthy volunteers but not to this magnitude. RAL is known to exhibit substantial interpatient variability and in this small study this could explain the substantial reduction. The present recommendations allow coadministration of BOC and RAL. I don't see a reason to change those, but I do believe a measure of caution and close monitoring is warranted until more data in patients are available.
(for FDA statement on boceprevir and protease inhibitors, see http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm291144.htm )
(for EMA statement on boceprevir and protease inhibitors, see
www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2012/02/news_detail_001444.jsp&mid=WC0b01ac058004d5c1 )
2. The effect of BOC and TVR on maraviroc (MVC).
Abstract O-17 described a drug-drug interaction study of BOC or TVR combined with MVC in male healthy volunteers. MVC was given at a dose of 150 mg twice daily; PK samples were obtained after 10 days of concomitant administration. Concentrations of MVC were increased by both BOC and TVR. The AUC for MVC was increased 3-fold by BOC and was increased 9.5-fold by TVR; MVC maximum and trough concentrations were similarly increased. These data provide additional evidence that TVR is more potent inhibitor of CYP3A than BOC, as seen in the interaction studies with midazolam, considered a pure CYP3A substrate, where a 9-fold increase was seen with TVR compared with a 5.3-fold increase with BOC. The magnitude of the increase in MVC concentrations with BOC is very similar to that seen with atazanavir 400 mg once daily (no ritonavir). The magnitude of the increase with TVR is greater than that with ketoconazole and essentially the same as seen with saquinavir/ritonavir. Concentrations of BOC and TVR were consistent with those seen in historical controls. In this short-term study in healthy volunteers no safety signal was seen that would preclude coadministration. The current recommendation for MVC dosing at 150 mg twice daily in the presence of strong CYP3A inhibitors also seems appropriate if given together with BOC or TVR.
3. The effect of BOC and TVR on dolutegravir (DTG) PK.
DTG PK were evaluated in 32 healthy volunteers when combined with either BOC or TVR. The DTG dose was 50 mg once daily, and usual doses of BOC and TVR were used. When given with BOC there was no change in DTG concentrations. TVR coadministration produced a 25% increase in the AUC of DTG, a change not considered clinically important. DTG did not change TVR concentrations; the data on BOC concentrations are not yet available. In this short-term study, no new adverse reactions or safety concerns were observed with these combinations. DTG can be combined with BOC or TVR without any dose adjustments.
This meeting continues to be a premier meeting focused on clinical pharmacologic issues in the treatment of HIV infection, with increasing attention on coinfection with TB and HCV. Furthermore, it remains a venue for scientific interactions among scientists from academia, pharmaceutical industry and regulatory authorities, which is an almost unique attribute. The 15th International Workshop on Clinical Pharmacology of HIV Therapy will be held in the United States in 2014. This meeting deserves strong support from industry, regulatory authorities, academia and clinicians.
|
| |
|
 |
 |
|
|