 |
 |
 |
| |
Clinical Pharmacology at CROI 2014
|
| |
| |
Courtney V. Fletcher, Pharm.D.
Dean and Professor
Anthony T. Podany, Pharm.D.
Post-Doctoral Fellow
College of Pharmacy
University of Nebraska Medical Center
986000 Nebraska Medical Center
Omaha, NE 68198
The 21st Conference on Retroviruses and Opportunistic Infections (CROI) was held in Boston, MA, from March 3 to March 6, 2014. CROI continues to be the premier HIV-focused scientific meeting. In this report we will highlight abstracts focused on pharmacologic issues that are of broad interest or might benefit from some expert clarification. Abstracts will be discussed in four broad categories: (i) the therapy of HIV infection, (ii) PrEP, (iii) advances in treatment of the TB-HIV coinfected person, and (iv) new drugs for treatment of HCV.
Abbreviations
%CV, percent coefficient of variation
ABC, abacavir
ACTG, adult AIDS clinical trials group
APV, amprenavir
ARV, antiretroviral drug
ART, antiretroviral drug therapy
AUC, area under the concentration-time curve
ATV, atazanavir
BID, twice daily
BOC, boceprevir
C12, drug concentration at 12 hours post dose
Cmax, maximum drug concentration
Cmin, minimum drug concentration
CVC, cenicriviroc
CNS, central nervous system
COBI, cobicistat
CSF, cerebrospinal fluid
CVF, Cervicovaginal fluid
Ctrough, concentration immediately before the next dose
CYP, cytochrome P450 drug metabolizing enzymes
DBS, dried blood spot
DCV, daclatasvir
DHHS, Department of Health and Human Services
DSMB, data safety monitoring board
DTG, dolutegravir
DRV, darunavir
ddI, didanosine
EFV, efavirenz
EVG, elvitegravir
FDV, faldaprevir
FTC, emtricitabine
ETR, etravirine
fAPV, fosamprenavir
GMR, geometric means ratio
HAND, HIV-associated neurocognitive disorders
HDAc, histone deacetylase
IC50, concentration of drug required to inhibit viral replication in vitro by 50%
IC90, concentration of drug required to inhibit viral replication in vitro by 90%
IDV, indinavir
IM, intramuscular
IQ, inhibitory quotient
IVR, intra-vaginal ring
3TC, lamivudine
LDV, ledipasvir
LPV, lopinavir
MVC, maraviroc
MPA, medroxyprogesterone acetate
NVP, nevirapine
NRTI, nucleoside reverse transcriptase inhibitor
NNRTI, non-nucleoside reverse transcriptase inhibitor
PACTG, pediatric AIDS clinical trials group
PBMCs, peripheral blood mononuclear cells
PD, pharmacodynamic
PEG-IFN, pegylated Interferon
PG, pharmacogenetics/pharmacogenomics
PK, pharmacokinetic
PI, inhibitor of HIV protease
PrEP, pre-exposure prophylaxis
QD, once daily
r or RTV, ritonavir
RAL, raltegravir
RBT, rifabutin
RBV, ribavirin
RPT, rifapentine
RIF, rifampin
RPV, rilpivirine
SQV, saquinavir
SC, subcutaneous
SOF, sofosbuvir
TAF, tenofovir alafenamide fumarate
TDF, tenofovir disoproxil fumarate
TFV, tenofovir
TVR, telaprevir
TDM, therapeutic drug monitoring
TPV, tipranavir
TB, tuberculosis
ZDV, zidovudine
I. The Pharmacotherapy of HIV Infection
1. 91LB. GSK1265744 (744) and rilpivirine (RPV) as 2-drug maintenance therapy. GSK-744 is an investigational integrase inhibitor being developed in 2 formulations, an oral tablet and a long-acting injectable (see also section II below). Likewise for RPV, there is already an approved in oral tablet formulation, and a long acting injectable suspension is under development (see Antimicrob Agents Chemother 2010;54:2042-50). In this study, ART-naïve persons were randomized to receive induction therapy with GSK-744 in an oral dose of 10, 30 or 60 mg once daily, or EFV, both in combination with TDF/FTC or ABC/3TC. At week 24, those receiving GSK-744 with HIV-RNA < 50 cpm discontinued the NRTIs and started RPV, 25mg once daily; the regimen for those receiving EFV was unchanged. At week 24, the % of subjects with HIV-RNA < 50 cpm was 86% for all doses of GSK-744 and 74% for EFV. At week 48, these results were 82% for GSK-744 (now just plus RPV) and 71% for EFV (plus 2 NRTIs). Ultimately, where these 2 companies want to go is to use their long-acting injectable formulations for maintenance therapy. These results provide a basis for that study. I'd just add a note of caution, that 24-week results don't necessarily predict success for years, so long-term evaluations are absolutely essential. Oral therapy with GSK-744 also seems viable, and the investigators stated that a dose of 30 mg once daily has been selected for further study.
2. 92LB. Doravirine (MK-1439), the investigational NNRTI from Merck. Doravirine (MK-1439) is an investigational NNRTI from Merck that has activity against wild-type HIV and strains with common NNRTI mutations. At CROI 2014, the results of a randomized (vs. EFV) dose ranging trial were reported in ART-naïve persons. MK-1439 doses of 25, 50, 100 and 200 mg once daily were studied. Both MK-1439 and EFV were combined with TDF/FTC. The primary endpoint was HIV-RNA < 40 cpm at week 24. The proportions of persons achieving these endpoints who received MK-1439 were: 25mg, 80%; 50mg, 76%; 100mg, 71% and 200mg, 78%. 64% of EFV recipients met the endpoint. Drug-related adverse events that led to treatment discontinuation occurred in 2% of MK-1439 recipients and 5% of EFV. MK-1439 is associated with CNS side effects, which were seen in 20% of participants across all 4 doses, and reported in 33% of EFV recipients [from Jules: this was an early study, these findings of CNS side effects may be an artifact (not real), future larger studies should provide clearer picture of CNS profile]. Abnormal dreams were the most common adverse event: 9% for MK-1439 (all doses) and 7% for EFV. The study investigators stated the 100 mg once daily dose of MK-1439 was selected to move forward in the next phase of clinical investigation. A few comments. I thought (as did others in the audience) the rate of EFV participants who reached the viral load endpoint wasn't what we usually expect (e.g. see abstract 91LB just discussed above where the rate was 74% for EFV). The presenting author didn't offer any explanation. We don't yet know the drug-interaction potential of MK-1439. At CROI 2013, (poster 527) a drug interaction study with midazolam showed that MK-1439 was not an inducer or inhibitor of CYP3A and thus seems to have a low potential to cause drug-interactions. What we don't know yet is whether it will be a victim of drug-interactions. Finally, MK-1439 obviously isn't devoid of CNS adverse effects. It remains to be shown whether this drug will have any advantages over EFV for the treatment-naïve person.
3. Abstract 514LB provides PK and safety support for administration of depot medroxyprogesterone acetate (MPA) in HIV+ women receiving LPV/r. Abstract 514LB reported the results of a drug-drug interaction study in 24 HIV-infected women receiving BID LPV/r (with 2 NRTIs) before and after a single 150 mg IM injection of depot MPA. There was no difference in the PK parameters of LPV and RTV before and 4 weeks after depot MPA. For example, median LPV trough concentrations were 5630 ng/mL before and 5700 ng/mL after. Overall, MPA concentrations, when compared with historical controls, were significantly higher in the presence of LPV/r: the 12-week AUC for MPA was 12.4 ng*wk/mL in controls compared with 18.1 ng*wk/mL with LPV/r. However, MPA concentrations 12-weeks after the injection were similar: 0.43 ng/mL in controls compared with 0.47 ng/mL with LPV/r. Adverse events were similar to historical controls. No woman ovulated over the 12-week study. Overall, because the 12-week MPA concentrations were very comparable, and safety and tolerance seemed no different from controls, these data support the usual dose of depot MPA (150 mg every 3 months) can be given to women taking the 400/100 dose of LPV/r BID. As the authors cautioned, don't extrapolate these findings to other ritonavir-boosted PIs. I would urge careful monitoring and that women report any side effects, changes in menstrual patterns to their health care provider - that said, these data indicate depot MPA is a contraception option for women taking LPV/r.
4. Tenofovir dosing in adults with moderate renal impairment - 150 mg once daily compared with 300 mg every 48 hours. The current dose recommendation for TDF in adults with moderate renal impairment [defined as a creatinine clearance (CLcr) between 30-49 mls/min] is 300 mg every 48 hours. The dose of FTC is also the usual dose (200 mg) every 48 hours for patients with CLcr 30-49 mls/min, which allows one TDF/FRTC tablet (e.g. Truvada) to be given every 48 hours. The TDF dose has received little evaluation in HIV-infected persons. If TDF is given with 3TC, the dose recommendation can present challenges because of different dosing schedules: the 3TC in patients with moderate renal impairment is half the usual dose once daily (e.g., 150mg QD).
Abstract 516 evaluated a TDF dose of 150 mg QD in 20 HIV-infected adults with moderate renal impairment receiving LPV/r and 3TC. Each subject underwent 2 PK studies, one while receiving TDF 300 mg every 48 hours, and a second while receiving 150 mg once daily. Although the Cmax was slightly lower and the trough concentration was slightly higher with the 150 mg QD dose, overall, there was no difference in the 48-hour AUC between these 2 TDF dosing schedules.
TDF needs to always be used with caution and close monitoring in adults with renal impairment and the dose must be adjusted for reduced CLcr. First, I think these data provide some reassurance about the appropriateness of the 300 mg every 48h dosing schedule in HIV-infected persons who have mild-moderate renal impairment. And second, the data provide a PK basis to indicate that 150 mg once daily will provide equivalent exposure. Tenofovir (Viread) is available in 150, 200, 250 and 300 mg tablets.
5. EFV PK in ENCORE1 - and is 1000 ng/mL still the suggested threshold concentration for EFV? ENCORE1 was a randomized, placebo controlled trial that compared an EFV dose of 400 mg QD with the usual 600 mg QD (both with TDF/FTC) in 630 ARV-naïve persons in 13 countries. The results were published Feb 10, 2014 in Lancet. Key findings were: no difference in the proportion of subjects at wk 48 with viral load < 200 cpm (400 mg, 94.1% vs 600 mg, 92.2%); no difference in the overall proportions of participants reporting adverse events or the severity of adverse events; no statistical difference in proportions with CNS adverse effects; and fewer patients in the 400 mg arm discontinued therapy from drug-related adverse events (400mg, n=6, 2% vs. 600mg, n=18, 6%).
Abstract 510 reported the results of an intensive PK study in 46 participants in ENCORE1.
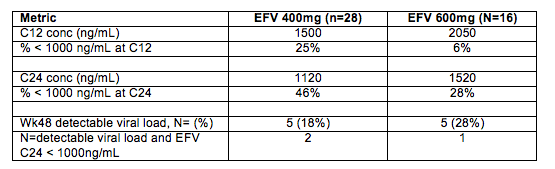
Some comments and thoughts. First, ENCORE1 used an endpoint of viral load < 200 copies/mL at week 48. This choice opens the question of whether EFV 400 mg could be equivalent to 600 mg in achieving suppression to < 200 cpm but not equivalent to < 50 cpm? Adherence monitoring was by self-reported adherence at weeks 4 and 48, and did not differ by study arm. The PrEP studies have shown (perhaps a bit painfully) how poor a measure self-reported adherence is (see Abstract 44 below). The PK data in Abstract 510 are consistent with expectations: when the EFV dose is reduced by 33% (600 to 400 mg), an approximate reduction in AUC, C12 and C24 of 30% is seen. Now a reminder about where the recommendation for 1000 ng/mL came from. The Marzolini study (AIDS 2001;15:71-75) found that 50% of patients (5/10) with a mid-interval EFV concentrations (average 14 hours post dose) < 1000 ng/mL had virologic failure compared with 22% (26/120) who had concentrations > 1000 ng/mL. It is also worth mentioning the concomitant therapy at use in 2001: the most common NRTI combination was ZDV+3TC, and nelfinavir was the most common concomitant PI. Today, backbone drugs (e.g. TDF/FTC as used in ENCORE1) are more potent and could contribute to the need for a little less EFV. Taking the ENCORE1 clinical trial and PK data at face value, I believe they provide compelling evidence to investigate further whether the suggested mid-interval target concentration for EFV of 1000 ng/mL should be reduced to perhaps 700 ng/mL (a 30% decrease)? But, lets do this as well as any dose reductions carefully. The losers in an ill-conceived race to lower doses are the patients - virologic failure and resistance have real consequences.
II. Pre-Exposure Prophylaxis (PrEP)
GSK1265744 (744). Without question the data presented on 744 for PrEP were one of the highlights of CROI 2014. Abstract 39 described the association between 744 plasma concentrations and prevention of rectal SHIV transmission to macaques. This study was published in the March 7 issue of Science (see paper at: pubmed, http://www.ncbi.nlm.nih.gov/pubmed/24594934; and
http://www.sciencemag.org/content/343/6175/1151.full.pdf; and commentary at http://www.sciencemag.org/content/343/6175/1067.full.pdf; registration/subscription required).
NATAP link- CROI: Long-Acting Integrase Inhibitor GSK744 for PrEP (Once Monthly or maybe longer) - (03/05/14) includes Science publication, link to oral presentation, slides
In this work, 8 macaques were given 2 IM injections of 744 at a dose of 50 mg/kg. The first was given 1 week before the first virus exposure and the second 4 weeks later. The macaques were challenged weekly with SHIV administered rectally for 8 weeks or until they became infected. 8 additional macaques were similarly challenged with SHIV but did not receive 744. All control animals became infected after a median of two SHIV challenges. All (8/8) macaques that received 744 remained uninfected (aviremic) throughout the challenge period.
In a second experiment, 12 macaques received only a single IM dose of 744 one week before SHIV exposure, and were again challenged weekly until infection was detected. Four macaques served as controls, and all quickly became infected after virus challenge. All treated animals remained uninfected for 8 weeks after the single 744 injection, and then all became infected in a pattern associated with the decline in 744 plasma concentrations. All infections occurred when 744 plasma concentrations were less than 0.50 μg/mL, a concentration approximately 3x greater than the protein binding adjusted concentration IC90 (PAIC90; 166 ng/mL). If 744 concentrations were >3X the PAIC90 values there were no infections in 59 challenges (0/59; 100% protection); with values between 1-3X the PAIC90 there was 1 infection (1/22; 95% protection); and there were 11 infections (11/43; 74% protection) when the 744 concentrations were <1X the PAIC90 values. These data much more precisely identify a target range for 744 concentrations.
The next steps are to translate these findings into humans, and of particular importance will be establishing the safety profile and optimal dosing interval given the wide variability in the elimination half-life of GSK 744 (21-50 days). Half-life is a key consideration because it defines how often the drug needs to be administered. Collectively, these data allow a real sense of optimism that GSK744 in humans may offer a very high degree of protection from HIV infection with perhaps every 12-week administration. If this can be achieved, it will overcome difficulties found in clinical trials with daily medication adherence; however, the requirement for multiple injections over a lifetime will present its own set of adherence challenges.
Complementary is Abstract 40LB, where 6 macaques were given 2 IM injections of 50 mg/kg GSK744, four weeks apart to evaluate concentrations in plasma and vaginal secretions. Maximum concentrations in vaginal secretions were on average 24% those in plasma, and the AUC over 28 days was 16% that of plasma. The protective effect of every 4-week GSK744 administration against twice weekly vaginal SHIV exposure was subsequently evaluated. All 6 macaques remained uninfected throughout 22 SHIV challenges and for 12 weeks after the last GSK744 injection. Though vaginal concentrations of GSK744 are lower than plasma, they were sufficient to provide a high degree of protection to vaginal exposure, and provide a basis to evaluate GSK744 as a PrEP agent for women.
CROI: Monthly GSK744 long-acting injections protect macaques against repeated vaginal SHIV exposures - (03/14/14)
CROI: GSK1265744 Long-Acting Protects Macaques against Repeated High-Dose Intravaginal Challenges & Depo Provera-treated - (03/14/14)
2. Dapivirine (DPV) vaginal ring, gel and film formulations for HIV prevention.
In Abstract 41, a vaginal ring containing 25 mg DPV was used for 28 days followed by 24 days off in 48 HIV negative sexually abstinent women. DPV could be measured in plasma, though at very low concentrations. At day 28 (last day of ring use), DPV was present in vaginal secretions and in tissue. In an ex vivo HIV challenge assay with fresh cervical tissue, the DPV concentrations in tissue (at 28 days after insertion of the ring) was able to block HIV infection and the degree of protection was associated with DPV concentrations in the tissue.
Abstract 42LB reported the results of a phase I safety and PKPD study of vaginal gel and film formulations of DPV in 60 HIV negative women randomized to receive placebo gel, DPV gel (0.05%), placebo film, and DPV film (1.25 mg). Participants used the assigned product daily for 7 days. Plasma DPV concentrations at the end of 7 days were comparable between the DPV gel and film; tissue concentrations of DPV, however, were 4x higher with the gel. An ex vivo HIV challenge of tissue obtained 2 hours after the last dose of gel or film found both products were protective, but the level of protection favored the gel formulation. Both products appeared safe. Some women had difficulty in proper placement of the film product.
These two abstracts indicate DPV vaginal ring, gel and film formulation may be viable strategies for HIV prevention.
Abstract 41 -
http://www.croiwebcasts.org/console/player/22068?mediaType=slideVideo&
Abstract 42LB -
http://www.croiwebcasts.org/console/player/22069?mediaType=slideVideo&
3. Integrase inhibitor vaginal gel formulations for HIV prevention. Though this work wasn't presented at CROI 2014, it was published in Science Translational Medicine (March 12; pubmed at: http://www.ncbi.nlm.nih.gov/pubmed/24622515) and adds to this discussion of new ARV drugs and formulations for HIV prevention. Raltegravir (RAL) and the integrase inhibitor L-870812 (L-812) were formulated into vaginal gel products. Female macaques were administered the gels vaginally and challenged with low-dose SHIV twice weekly for up to 10 weeks. The gels were given 30 minutes prior to SHIV challenge to evaluate pre-exposure efficacy (PrEP) and 3 hours after SHIV challenge to evaluate post-exposure efficacy (PEP). Two of the 3 macaques that received L-812 for PrEP remained uninfected after 14 challenges; the breakthrough infection occurred after 9 challenges. Five of 6 macaques that received RAL for PEP remained uninfected after 20 SHIV challenges and the 10-week follow-up period. These studies provide evidence that topically applied integrase inhibitors can prevent SHIV infection whether given pre or post exposure. The post exposure activity may arise from the difference mechanism of action that integrase inhibitors have, working late in the viral life cycle, versus NRTIs that work early. If this strategy is proven effective in humans, PEP administration of an integrase inhibitor vaginal gel may be another approach to improving acceptability and adherence.
Download the PDF here
4. Measuring adherence - better and real-time methods are urgently needed. In work from the VOICE trial, various behavioral measures of adherence were compared with measurement of plasma and vaginal swab concentrations of TFV. The behavioral tools used were face-to-face interviews and monthly clinic product counts and audio-computer assisted self-interview conducted quarterly. When compared with measured concentrations, which provides an objective indication of drug or product use, none of the behavioral methods correctly predicted non-adherence. I've commented in these reports before that it does absolutely no good to identify non-adherence after the fact - and agree with these authors that we urgently need tools that can easily and inexpensively be used in real-time to identify non-adherence and a support structure for study participants and patients that can implement interventions to improve adherence.
III. Advances in the Treatment of Tuberculosis in HIV-TB Co-infected Persons.
There was an abundance of new data on the treatment of HIV and TB co-infected individuals. Much focused on results of drug-drug interaction studies designed to evaluate the effects of anti-tuberculosis therapy on ART. Abstract #496 investigated the PK of raltegravir (RAL) when given with intermittent rifampin (RIF) anti-TB therapy in 18 healthy volunteers; RAL 400mg BID and 800mg BID when given with RIF 900mg thrice weekly were compared. RAL trough concentrations were significantly lower at 400mg compared with 800mg BID in the presence of RIF. Data from the previously completed QDMRK study have shown RAL trough concentrations to be a strong predictor of virologic failure. The authors recommend using a RAL dose of 800mg BID when dosing with daily or intermittent RIF anti-tuberculosis therapy. We agree with this recommendation.
Two abstracts investigated the effects of RIF TB therapy on EFV PK. Abstract 494 presented interaction data between EFV and RIF based TB treatment from 42 individuals in the STRIDE study. Interestingly, RIF based TB treatment was associated with increased EFV trough concentrations. EFV concentrations were highest in poor CYP2B6 metabolizers and slow NAT acetylators, suggesting that isoniazid therapy, given concurrently with RIF, may have more interaction potential than just RIF in certain genotype individuals.
Abstract 495 investigated RIF and EFV interactions in 62 Mozambican HIV-TB co-infected adults. Interactions between TB treatment with RIF, isoniazid, ethambutol and pyrazinamide along with EFV also showed higher EFV concentrations while on the TB treatment. These data agree with the STRIDE data indicating the CYP inductive effects of RIF may be counteracted by inhibition effects of isoniazid on the CYP2A6 metabolizing pathway of EFV in certain genotype individuals. At minimum, these data depict the necessity to investigate further the effects of isoniazid on EFV metabolism; it is looking very clear that the rifamycin antibiotics are only part of the interaction algorithm.
A second rifamycin antibiotic receiving attention at CROI 2014 was rifapentine (RPT). RPT is advantageous to use in the treatment of TB as its long half-life lends to less frequent dosing and its potency allows for shorter durations of treatment for both latent and active TB disease. Abstract 493 presented a PK interaction study between RPT, 900mg once weekly for 3 weeks, and Atripla (EFV, FTC, TDF). Results from 12 HIV+ individuals free from TB showed that once weekly RPT administration does not significantly alter the PK of EFV, FTC or TFV.
Abstract #105 reported the results of a PK study from ACTG A5279, a study of HIV+ individuals who either have latent TB or live in a high burden area of TB. The PK study reported interaction data between weight based RPT (also given with isoniazid) administered daily for 4 weeks in individuals on EFV-based ART. In 87 patients, the oral clearance of EFV was not significantly increased over the 4 weeks of daily RPT therapy. These data provided evidence that a daily RPT for 4 weeks as treatment of latent TB is safe to use with EFV-based ART.
IV. New Drugs for Hepatitis C
Emerging data on the treatment of hepatitis C virus (HCV) infection was a real highlight of CROI 2014. It is an exciting time with new drugs being developed and the possibility of interferon free regimens with high cure rates appear to be not far into the future. One such agent reported on at this year's conference was faldaprevir (FDV). FDV is a potent NS3/4A protease inhibitor and is being studied in both HCV mono-infected as well as HIV/HCV co-infected individuals. FDV is a CYP3A4 substrate and inhibitor and thus has a high interaction potential with several ARVs. FDV's inhibition of CY3A4 appears to be dose dependent, with the 240mg dose showing stronger inhibition than the 120mg dose (Sabo J,et al. ICAAC 2012; Poster A-1248). There were 3 posters presented this year that describe possible interactions between FDV and ART.
Abstracts 497 and 499 described results of PK studies within the STARTVerso4 trial investigating the interactions between FDV and EFV, DRV, ATV, RAL and TDF. The trial included 308 HIV+ patients with genotype 1 HCV. Patients taking DRV/r or ATV/r based ART received 120mg of FDV daily while patients receiving EFV based ART received 240mg of FDV daily. The basis for this was studies presented at CROI 2013 that showed FDV concentrations were increased 130% when given with DRV/r and were decreased 35% when given with EFV. (see CROI 2013 Abstracts 35 and 40LB; http://www.natap.org/2013/CROI/croi_03.htm
http://www.natap.org/2013/CROI/croi_02.htm )
Patients on any other ART regimen were randomly assigned one of the treatment doses of FDV (120 or 240mg). The authors concluded their data support the use of 120mg FDV when used in combination with ART containing RAL, DRV/r or ATV/r, and FDV at 240mg when given with EFV containing ART.
CROI 2014: PHARMACOKINETICS OF FALDAPREVIR AND ANTIRETROVIRALS IN PATIENTS WITH HIV/HCV CO-INFECTION - (03/21/14)
CROI 2014: EFFECT OF FALDAPREVIR ON ATAZANAVIR PHARMACOKINETICS IN PATIENTS WITH HIV/HCV CO-INFECTION - (03/21/14)
CROI 2014: EFFECT OF FALDAPREVIR ON RALTEGRAVIR PHARMACOKINETICS IN HEALTHY VOLUNTEERS - (03/21/14)
One note of caution regarding DRV concentrations. In healthy volunteers, DRV trough concentrations were decreased approximately 12% (CROI 2013, Abstract 35). In the HIV-HCV infected persons in this study, the DRV trough concentrations were decreased 50%: from 4123 ng/mL without FDV to 2310 ng/mL in the presence of FDV. While the authors stated there were no HIV treatment failures (Abstract 23), we would suggest caution and close monitoring of HIV response if combining FDV and DRV/r, especially in HIV treatment-experienced persons.
Full details of these abstracts can be found at: http://croiconference.org/sites/all/abstracts/497.pdf and http://croiconference.org/sites/all/abstracts/499.pdf
Abstract 501 further investigated the PK of RAL when given with FDV in 24 healthy volunteers. The authors studied a FDV dose of 240mg daily (the highest dose in clinical development) with a RAL dose of 400mg BID. Both RAL and RAL-glucuronide exposures (AUC, Cmax) were approximately 2.5 fold higher when given with FDV 240mg than when given alone. The authors concluded the interaction between RAL and FDV 240mg is probably driven by transporters more so than metabolism. Adverse events and safety profiles of the individuals in the study were similar to profiles of the individual agents alone. Referring back to abstract 497, studies in HIV/HCV co-infected individuals support the use of 120mg FDV when used with RAL. We hope the exact mechanism/transporter of this interaction can be identified as knowing that would help predict other interactions.
Abstract 23 reported the efficacy results of the STARTVerso4 trial which investigated FDV + pegylated interferon (PEG-IFN) + ribavirin (RBV) in HIV/HCV co-infected individuals. Treatment arms were either FDV 120mg for 12 weeks or FDV 240mg for 12 or 24 weeks. 96% of the patients in the study were on ART. Patients on a PI regimen were assigned to the 120mg FDV dose while those on EFV based regimens were assigned to the 240mg FDV dose, all other regimens were randomized between the FDV doses. SVR4 were 72-84% across the FDV doses/durations. Continuation of PEG-IFN and RBV for an additional 24 weeks appeared to have minimal benefit as measured by SVR4 (92% vs 95%). As stated during the presentation, SVR12 (study endpoint) was achieved in 71% of subjects receiving FDV at 120mg and 72% of those receiving 240mg. These similar efficacy results, along with the similar safety profiles to those seen in the HCV mono-infection studies of FDV suggest that FDV+PEG-IFN+RBV will likely be an option for treatment of genotype 1 HCV individuals co-infected with HIV.
CROI: SVR12 72% With Faldaprevir Plus PegIFN/RBV in Naives With HCV/HIV - (03/04/14)
A second investigational agent for HCV reported at CROI was daclatasvir (DCV). DCV is a novel NS5A inhibitor. Abstract 502 investigated the potential interactions between DCV and cyclosporine or tacrolimus. Recurrence rates of HCV are very high post transplant and there lies a need for HCV drugs that can be used with immunosuppression regimens post-transplant. In this study of 14 healthy volunteers per treatment group, no clinically relevant steady state drug interactions between cyclosporine and DCV or tacrolimus and DCV were found. Cmax and AUC values of the immunosuppressive agents were not changed more than 5% by addition of DCV as measured by geometric mean ratios. DCV trough concentrations were increased by 56% and 10% by cyclosporine and tacrolimus respectively. These data would indicate dose adjustments are unlikely to be necessary, but this must be confirmed in HCV infected persons.
Abstract 500 described PK interaction studies with the novel NS3/4A protease inhibitor MK-5172. The study enrolled 24 healthy volunteers and investigated a once daily dose of MK-5172 at 200mg when given with either RAL 400mg BID or TDF 300mg QD. Co-administration of TDF with MK-5172 resulted in minimally increased steady state exposure of TDF with geometric mean ratios of 1.18, 1.14 and 1.24 for AUC, Cmax and C24 respectively. TDF had no significant effect on steady state MK-5172 concentrations. These data provide evidence that MK-5172 can safely be administered with either RAL or TDF.
Two abstracts investigated interactions with the investigational NS5A inhibitor MK-8742. Abstract 638 detailed a two-way interaction study between MK-8742 and ritonavir boosted PI's. The PI's studied were: ATV/RTV 300/100mg daily, LPV/RTV 400/100 BID, and DRV/RTV 600/100 BID. MK-8742 had no significant effect on the exposures of any of the PIs as measured by AUC, Cmax and C24. The PI's, however, did alter the exposure of MK-8742. Exposures of MK-8742 increased from 2 to 5 fold when dosed with the PIs. ATV/RTV appeared to have the largest effect followed by LPV/RTV and DRV/RTV. Abstract 498 reported results of a similar two-way interaction study between MK-8742 and TDF, RAL and EFV. MK-8742 was found not to significantly alter the exposure of EFV or RAL while it increased the exposure of TDF as measured by AUC (+34%), Cmax (+47%) and C24 (+29%). Effects of the ARVs on MK-8742 were also mixed with TDF and RAL having no effect on MK-8742 exposure while EFV decreased MK-8742 AUC by 54%. Ongoing phase 2b studies of MK-8742 are ongoing and will yield more information on the safety of the drug in the treatment of HCV infection. Collectively, these abstracts suggest MK-8742 could be a feasible option for HIV/HCV co-infected individuals on PI or RAL based ART. However, EFV containing ART regimens appear to not be an option at this time until further dosing studies and efficacy studies can be performed.
All in all, CROI 2014 was an outstanding meeting, and progress on several fronts was reported. CROI 2015 will be in Seattle, Washington, February 23-26, 2015.
|
| |
|
 |
 |
|
|