 |
 |
 |
| |
HIV-Immune Complex Kidney Disease: Risk Factors and Progression to End-Stage Kidney Disease
|
| |
| |
Reported by Jules Levin
CROI 2014 March 3-6 Boston, MA
Booth JW1, Hamzah L2, Jose S1, McAdoo SB3, Kumar EA3, Williams D4, Kingdon E4, Watson J5, Khatib N5, Baharani J5, Mackie NE3, Levy JB3, Hendry BM2, Larbalestier N2, Hilton R2, Horsfield C2, O'Donnell PJ2, Jones R3, Williams I1, Johnson M1, Connolly J1, Sabin C1, Post FA2 1UCL Medical School/Royal Free Hospital, London, 2Kings College Hospital/Guy's and St Thomas' Hospital/King's College London, London, 3Imperial College/Chelsea and Westminster Hospital, London, 4Brighton and Sussex University Hospitals, Brighton, 5Heartlands Hospital, Birmingham, UK

Program Abstract
Background: Immune complex kidney disease (ICKD) has become the dominant pathology in renal biopsy series of HIV+ patients. The natural history of
ICKD and risk factors for ICKD remain poorly studied.
Methodology: We reviewed consecutive renal biopsies (1998-2012) of HIV+ patients attending eight clinics in the UK. ICKD was defined by the
unequivocal presence of glomerular immunoglobulin deposits and corroborated, where available, by the presence of electron dense deposits on electron
microscopy. We compared patients with ICKD to those with HIV-associated nephropathy (HIVAN) and patients in the UK CHIC cohort. Kaplan-Meier analysis
was used to estimate progression to end-stage kidney disease (ESKD), and Poisson regression analysis to examine factors associated with ICKD and
HIVAN in the UK CHIC cohort.
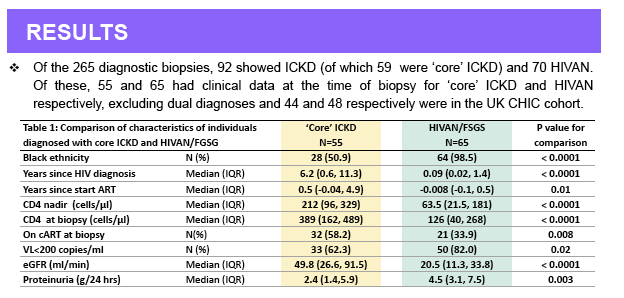
Results: Of the 250 diagnostic biopsies, 88 showed ICKD and 67 HIVAN. ICKD comprised a spectrum of patterns including membranous (n=17),
membrano-proliferative (n=5) and ICKD-not otherwise specified [NOS] (n=34); these groups displayed considerable overlap in both glomerular
morphology and location of deposits and were hence considered together as 'core' ICKD. The remaining ICKD biopsies showed IgA nephropathy (n= 26)
and lupus (n=6) nephropathy; these patients were analyzed separately and excluded from the present analyses. Patients with ICKD and HIVAN differed
by ethnicity (black: 54% vs. 98%), median known duration of HIV (6.2 vs. 0.1 years), degree of immunodeficiency (median CD4 nadir 155 vs. 54, median
CD4 at biopsy 382 vs. 116 cells/mm3) and severity of kidney disease at biopsy (median eGFR 53 vs. 22 mL/min/1.73m2) (p<0.001 for all). At biopsy, 59%
vs. 41% of patients had initiated combination antiretroviral therapy (ART) (p=0.01) and 66% vs. 34% had HIV RNA <200 c/mL (p=0.0008); at 1 and 5
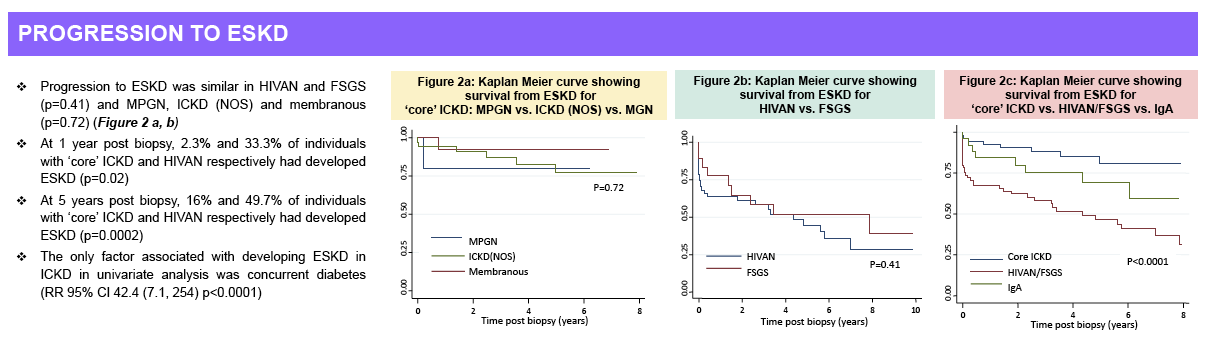
years post-biopsy, 4% vs. 29% and 13% vs. 45% of patients had progressed to ESKD (p<0.0001 for both).
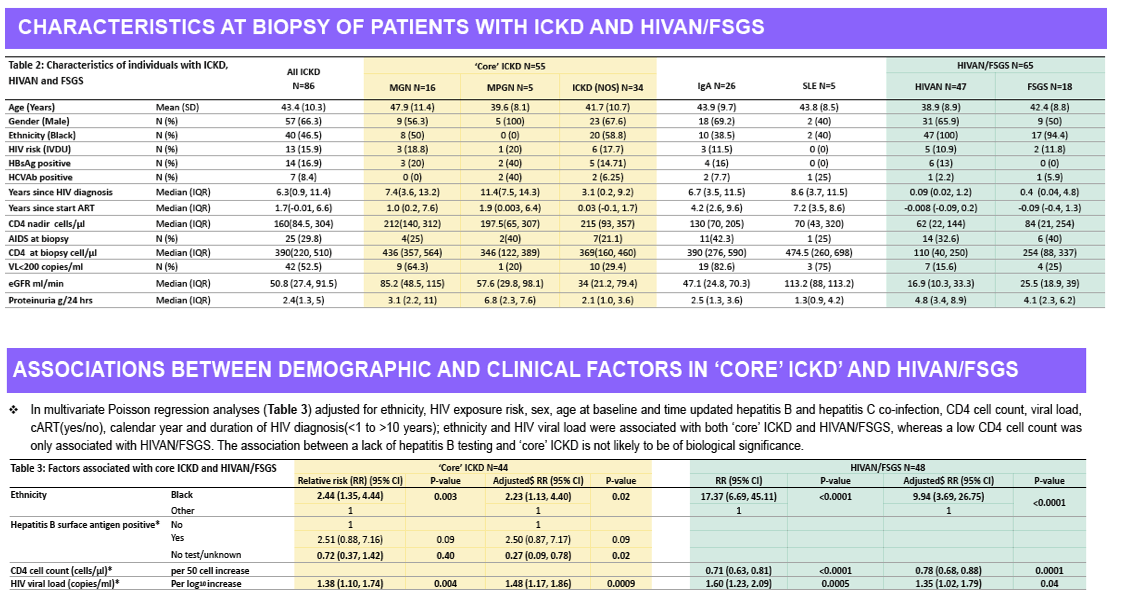
Of the 31,483 patients in the UK CHIC cohort, 32 developed HIVAN and 44 ICKD during follow up. In multivariable analyses, black ethnicity (IRR 2.23 [1.13, 4.40])
and HIV viraemia (1.48 [1.17, 1.86] per log10 increase in HIV RNA) were associated with 'core' ICKD, and black ethnicity (IRR 9.94 [3.69, 26.75]),
HIV viraemia (1.35 [1.02, 1.79]), and current CD4 cell count (0.78 [0.68, 0.88] per 50 cell increase) with HIVAN.
Conclusions: This is the first study to demonstrate a relationship between HIV replication and ICKD. Compared to HIVAN, ICKD was associated with less
advanced immunodeficiency and a lower rate of progression to ESKD. The observed association with HIV viraemia for both 'core' ICKD and HIVAN may
imply a pathogenetic role of HIV replication and its associated immune activation; it also suggests that suppressive ART may reduce the risk of developing
these types of kidney disease.

|
| |
|
 |
 |
|
|