 |
 |
 |
| |
Prolonged therapy of hepatitis delta for 96 weeks with PEG-IFNa-2a plus tenofovir or placebo does not prevent HDV RNA relapse: The HIDIT-2 study.
|
| |
| |
Reported by Jules Levin
EASL 2014 April 9-13 London, UK
Heiner Wedemeyer*, Cihan Yurdaydn*, Stefanie Ernst, Florin Alexandru Caruntu; Manuela G.Curescu, Kendal Yalcin, Ulus S. Akarca, Selim Gurel, Stefan Zeuzem, Andreas Erhardt, Stefan Luth, George V. Papatheodoridis, Onur Keskin, Kerstin Port, Monica Radu, Mustafa K. Celen, Ramazan dilman, Judith Stift, Benjamin Heidrich, Ingmar Mederacke, Svenja Hardtke, Armin Koch, Hans Peter Dienes, Michael P.Manns for the HIDIT-2 Study Group
*Cihan Yurdaydin and Heiner Wedemeyer contributed equally
Program abstract:
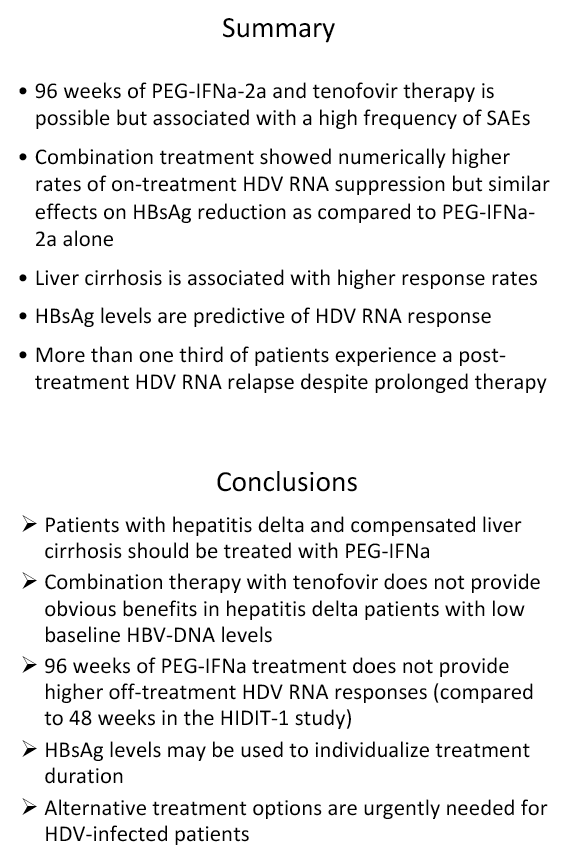
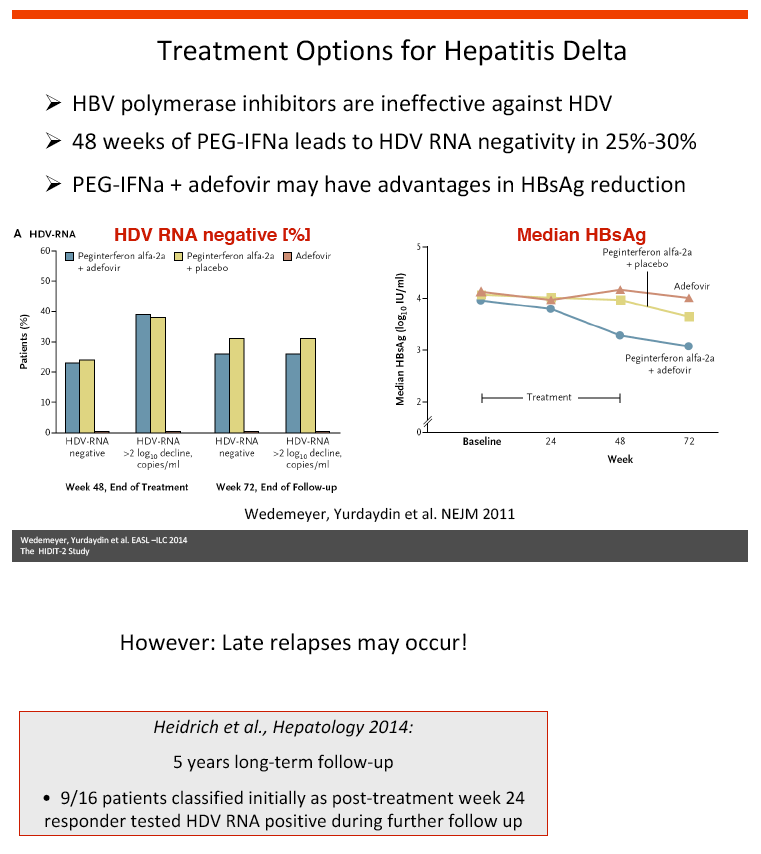
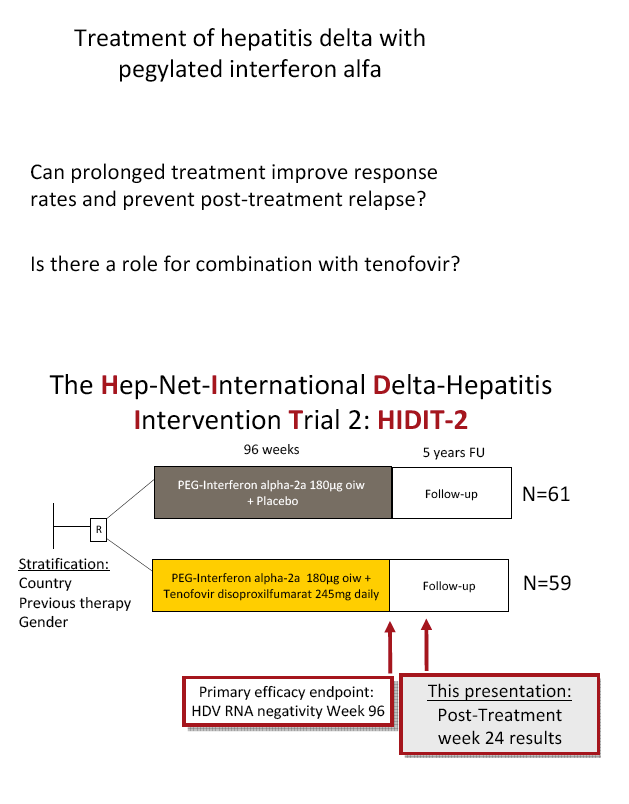
Background and aims: Hepatitis delta is the most severe form of chronic viral hepatitis. Pegylated interferon alfa for 1.0-1.5 years is effective in 25-30% of patients. The aim of the investigator-initiated HIDIT-2 trial was to investigate the efficacy of 96 weeks of PEG-IFNa-2a therapy plus tenofovir or placebo in patients with hepatitis delta. We here report the efficacy analysis after 24 weeks after the end of treatment.
Methods: 120 HDV-RNA-positive patients were recruited in 4 countries (Turkey n=50, Germany n=46, Romania n=19, Greece n=5). Patients were randomized to receive either 180 µg PEG-IFNa-2a weekly plus tenofovir disoproxil fumarate (300mg once daily; TFVarm A, n=59) or plus placebo (PBOarm B; n=61).
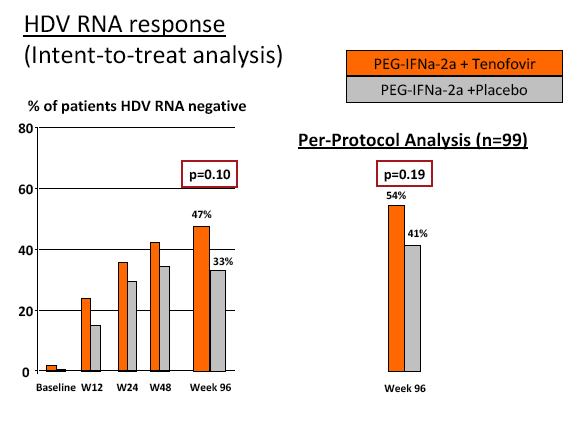
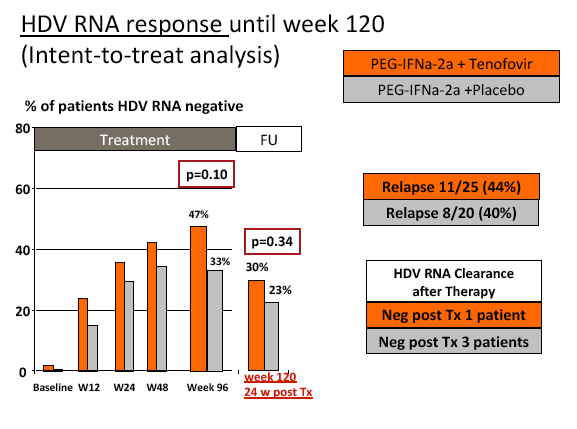
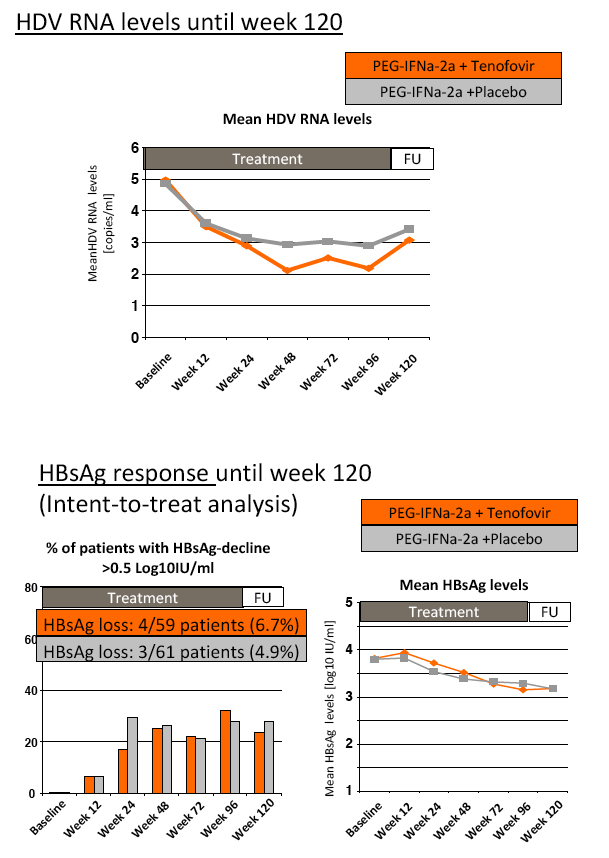
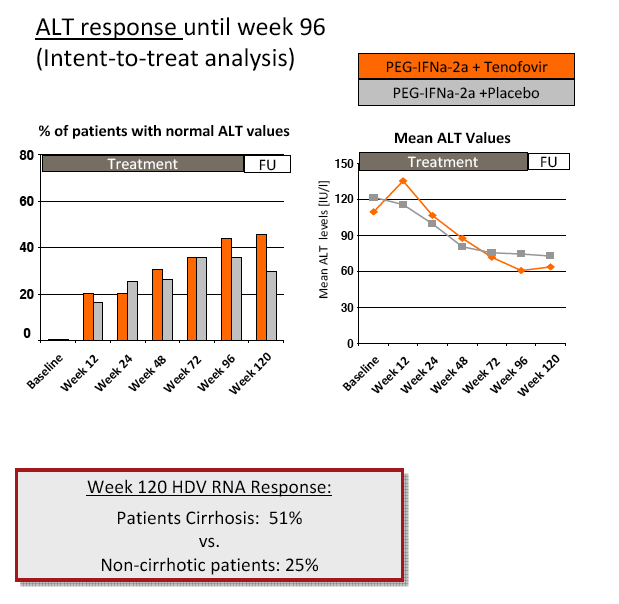
Results: The mean age was 40±11 years and 54 patients (45%) had liver cirrhosis. At the end of treatment HDV RNA was negative in 28 patients (48%) receiving combination therapy and in 20 patients (33%) receiving PEG-IFNa-2a and placebo (ITT analysis). Post-treatment, 36% and 39% of patients experienced an HDV RNA relapse while 1 and 6 patients became HDV RNA undetectable after treatment was stopped. The post-treatment week 24 HDV responses were 29% in tenofovir-treated patients and 21% in the placebo arm. Lower baseline HDV RNA and HBsAg levels were associated with the week 120 HDV RNA response. HBsAg was negative in 7 patients (3 TFVarm; 4 PBO).
Conclusions: Prolonged administration of PEG-IFNa-2a and tenofovir for 96 weeks does not prevent post-treatment relapse in HDV-infected patients. 48 weeks of PEG-IFNa-2a remains the standard therapy of hepatitis delta. Alternative treatment strategies are urgently needed.


|
| |
|
 |
 |
|
|