| |
Averting Hepatocellular Carcinoma in Chronic Hepatitis B With Antiviral Therapy: Tipping the Balance or Not Yet?
|
| |
| |
Download the PDF here
Gastroenterology
July 2014
HARRY L. A. JANSSEN
Toronto Center for Liver Diseases, Toronto Western and General Hospital, University Health Network, University of Toronto Toronto, Canada and Department of Gastroenterology and Hepatology Erasmus MC University Hospital Rotterdam Rotterdam, the Netherlands
AMBREEN ARIF
Toronto Center for Liver Diseases Toronto Western and General Hospital University Health Network University of Toronto Toronto, Canada
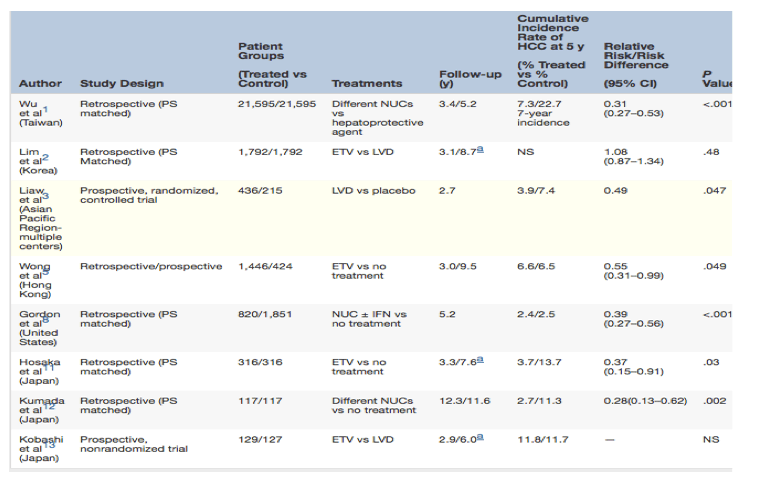
"We know that it is possible to almost fully prevent hepatic decompensation and portal hypertension in compensated cirrhosis with long-term NUCs, in particular entecavir and tenofovir. However, this is not the case for HCC, which can probably be reduced but definitely not fully prevented in HBV cirrhosis. A summary of the full papers published on HCC risk in HBV patients treated with NUCs is given in Table 1."
One of the most severe complications of chronic hepatitis B virus (HBV) infection is the development of hepatocellular carcinoma (HCC). The incidence of HCC in Western countries has risen considerably in the recent years and latest statistics from World Health Organization on trends in cancer incidence and mortality show that liver cancer has moved up to one of the leading causes of death worldwide.
Licensed oral antiviral agents for chronic HBV infection are the nucleos(t)ide analogs (NUCs), lamivudine, adefovir, telbivudine, entecavir, and tenofovir. Initially, goals in terms of treatment success were decline of HBV DNA levels but with more potent drugs and longer treatment duration, milestones for treatment have become more solid and clinically relevant. Reducing the risk of HCC is one of these milestones. NUC therapy seems to be beneficial in this regard and articles by Wu et al1 and Lim et al2 in this issue of Gastroenterology deliver stimulating evidence rendering feasible benefits for prevention of HCC secondary to treatment with NUCs.
The article by Wu et al1 shows the impact of NUC induced risk reduction for HCC in a large, nationwide cohort of chronic HBV patients. This retrospective study from Taiwan analyzed the risk of HCC in patients treated with either lamivudine, entecavir, telbivudine, or >1 NUC for ≥90 days, whereas the control population received a hepatoprotective agent only. Propensity scores were used to match the populations. The NUC-treated cohort had a significantly lower 7-year incidence of HCC compared with controls. The large cohort contributed to the strength of the study making it possible to assess the various subgroups and to identify which group amongst them benefited the most in terms of reduced risk of HCC.
Overall, the effect of NUCs on reduced risk for HCC was stronger in young patients without cirrhosis and in patients without diabetes mellitus. The major limitation of this study is that it was based on retrospective observational data and may be subject to selection bias and confounding factors. Another limitation is the absence of baseline information regarding alanine aminotransferase and HBV DNA levels, which makes calculation of HCC risk using the recently developed HCC scores impossible.
The second article by Lim et al2 is also a retrospective analysis of data comparing mid-term clinical outcome of chronic HBV patients, treated with entecavir or lamivudine at tertiary care hospital in Seoul, Korea. This study, also using propensity score matched cohorts, shows that patients in entecavir group had a significantly lower risk of death or transplantation than those treated with lamivudine, but there was no difference in risk for HCC between the 2 treatment groups. The paper provides further evidence for the persistent risk of HCC in HBV patients treated with even the most potent agents. The strengths of this study were its relatively large size and the fact that follow-up was rather complete with limited missing data. Major limitation of this study was the shorter duration of treatment and follow-up in the entecavir group, which may have prevented the authors from detecting a significant difference in reduced risk of HCC between this group and lamivudine. Use of rescue therapy with add-on adefovir in the lamivudine treated group is another potential explanation due to which treatment with entecavir did not reduce the HCC risk to a greater extent than lamivudine. Lastly, this was a single-center study, which limits the generalizability of the results.
The best way to assess the ability of reducing HCC with antiviral therapy is to perform a prospective, randomized, controlled trial. Liaw et al3 performed such a study about 10 years ago. In this study, patients were randomized to either lamivudine or placebo. The study reported a clear reduction in the composite endpoint of liver complications (cirrhosis, liver failure, and HCC) in the lamivudine group compared with placebo. Although this study suggested a reduction in the incidence of HCC within the lamivudine treated group, it was not significant after the analysis was corrected for the time to diagnosis of cancer (P =.052). The evidence that antiviral therapy is beneficial to reduce and even reverse cirrhosis4 and liver failure5 is currently so compelling that it is not considered ethical anymore to repeat such a placebo-controlled study with HCC as an endpoint. This could potentially be done in an immunotolerant patient with a very high viral load and no inflammation, but according to the current guidelines therapy is not recommended in these patients. It is known that several of them will develop active inflammation and even HCC later in life. However, because the number of immunotolerant patients developing HCC is limited, a power analysis of such a study would probably dictate huge numbers of patients with lengthy follow-up.
We know that it is possible to almost fully prevent hepatic decompensation and portal hypertension in compensated cirrhosis with long-term NUCs, in particular entecavir and tenofovir. However, this is not the case for HCC, which can probably be reduced but definitely not fully prevented in HBV cirrhosis. A summary of the full papers published on HCC risk in HBV patients treated with NUCs is given in Table 1.
Table 1. Literature on HBV-Related HCC Prevention by NUCs (Full Original Papers Only)
|
|
| |
| |
|
|
|