| |
Bristol-Myers Squibb to Present Data for Daclatasvir in Multiple Investigational All-oral Combinations across Hepatitis C Genotypes at The International Liver CongressTM
|
| |
| |
Daclatasvir data demonstrates potential to address high unmet needs, including cirrhotic and treatment-experienced patients, and those with genotypes 1, 2, 3 and 4
Breadth of viral hepatitis data underscores Company's commitment to advancing research of liver diseases
Category: R&D News
Monday, March 24, 2014 5:39 am EDT
"These results are encouraging and show the potential of daclatasvir across multiple treatment regimens, with the goal of helping patients achieve cure regardless of genotype, stage of disease or response to previous therapies"
PRINCETON, N.J.--(BUSINESS WIRE)--Bristol-Myers Squibb Company (NYSE:BMY) announced today that 12 abstracts have been accepted for presentation at The International Liver CongressTM, the 49th annual meeting of the European Association for the Study of the Liver (EASL), in London, April 9 - 13.
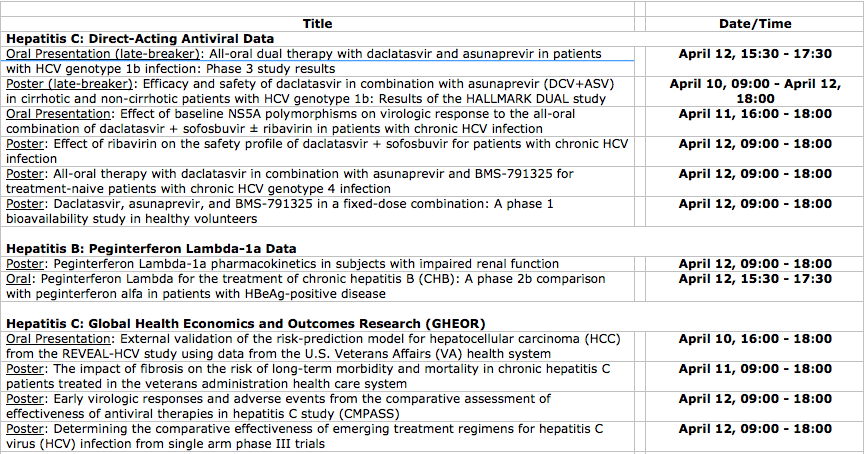
Key presentations include:
· Two sets of pivotal results from a global, Phase III study (HALLMARK DUAL) investigating the efficacy and safety of an all-oral, interferon- and ribavirin-free regimen of daclatasvir and asunaprevir, including data in cirrhotic and non-cirrhotic patients with HCV genotype 1b infection, will be presented as late-breakers.
· Virologic response results from analyses investigating daclatasvir in combination with sofosbuvir across genotypes 1, 2 and 3.
· Virologic response and safety data for the investigational all-oral 3DAA regimen (daclatasvir/asunaprevir/BMS-791325) in genotype 4 patients, as well as bioequivalence data for the daclatasvir 3DAA regimen, which is being studied as a fixed-dose-combination treatment with twice daily dosing.
"These results are encouraging and show the potential of daclatasvir across multiple treatment regimens, with the goal of helping patients achieve cure regardless of genotype, stage of disease or response to previous therapies," said Brian Daniels, MD, senior vice president, Global Development and Medical Affairs, Research and Development, Bristol-Myers Squibb. "The wealth of data we are sharing at the International Liver Congress continue the positive momentum for daclatasvir after the Marketing Authorization Application was validated for Accelerated Regulatory Review by the European Medicines Agency, highlighting the important potential role for daclatasvir-based regimens in Europe."
Bristol-Myers Squibb is studying a broad portfolio of compounds in hopes of providing flexible treatment options to address the diverse unmet medical needs of a global HCV patient population. These investigational compounds include daclatasvir, an investigational NS5A replication complex inhibitor that has shown high antiviral potency and pan-genotypic activity across HCV genotypes in vitro; asunaprevir, an investigational NS3 protease inhibitor; BMS-791325, an investigational non-nucleoside inhibitor of the NS5B polymerase; and peginterferon lambda-1a (Lambda), an investigational type III interferon that has the potential to offer an alternative to alfa-interferon.
The complete list of Bristol-Myers Squibb data presentations is below. Abstracts can be accessed on the ILC/EASL website at http://www.ilc-congress.eu.
About Hepatitis C
Hepatitis C is a virus that infects the liver and is transmitted through direct contact with infected blood and blood products. Up to 90 percent of those infected with hepatitis C will not spontaneously clear the virus and will become chronically infected. According to the World Health Organization, up to 20 percent of people with chronic hepatitis C will develop cirrhosis; of those, up to 25 percent may progress to liver cancer. In the European Union (EU) an estimated 9 million people are living with hepatitis C, and an estimated 170 million people worldwide are infected with the virus.
About Bristol-Myers Squibb's HCV Portfolio
Bristol-Myers Squibb's research efforts are focused on advancing late-stage compounds to deliver the most value to patients with hepatitis C. At the core of our pipeline is daclatasvir (DCV), an investigational NS5A replication complex inhibitor that has been studied in more than 5,500 patients as part of multiple direct-acting antiviral (DAA) based combination therapies. DCV has shown a low drug-drug interaction profile, supporting its potential use in multiple treatment regimens and in people with co-morbidities.
In 2014, the U.S. Food and Drug Administration (FDA) granted Bristol-Myers Squibb's investigational DCV Dual Regimen (daclatasvir and asunaprevir) Breakthrough Therapy Designation for use as a combination therapy in the treatment of genotype 1b HCV infection.
In 2013, Bristol-Myers Squibb's investigational all-oral 3DAA Regimen (daclatasvir/asunaprevir/BMS-791325) also received Breakthrough Therapy Designation, which helped to expedite the start of the ongoing Phase III UNITY Program. Study populations include non-cirrhotic na•ve, cirrhotic na•ve and previously treated patients. The daclatasvir 3DAA regimen is being studied as a fixed-dose-combination treatment with twice daily dosing.
Daclatasvir is also being investigated in combination with sofosbuvir in high unmet need patients, such as pre- and post-transplant patients, HIV/HCV co-infected patients, and patients with genotype 3, as part of the ongoing Phase III ALLY Program.
About Bristol-Myers Squibb
Bristol-Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information, please visit http://www.bms.com or follow us on Twitter at http://twitter.com/bmsnews.
|
|
| |
| |
|
|
|