| |
Treating HCV Earlier vs at Cirrhosis Reduces Serious Liver Disease Outcomes
|
| |
| |
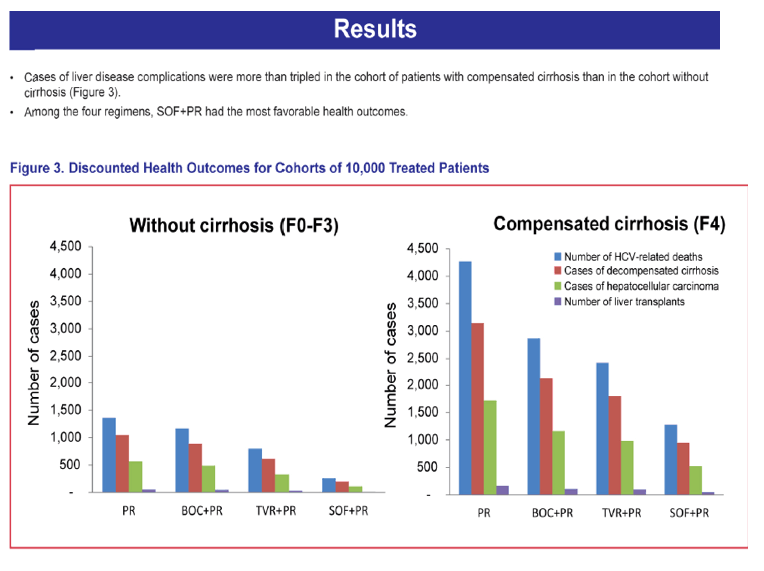
"Cases of liver disease complications (deaths/decompensated cirrhosis, HCC, transplants) more than tripled in patients with cirrhosis vs without cirrhosis"
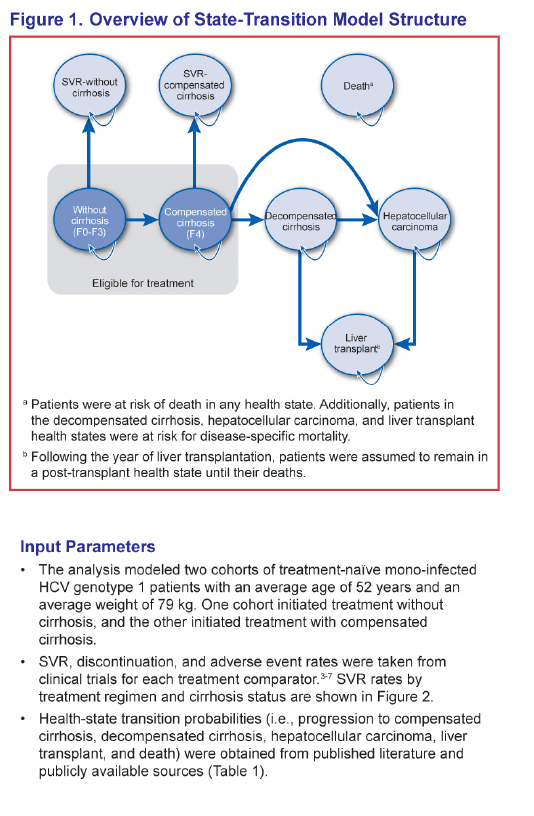
Study Title: Evaluation of the Long-Term Health Outcomes Associated with Earlier Versus Later Initiation of Treatment in Previously Untreated Patients with Chronic Hepatitis C Virus Genotype 1 Infection......"Cases of liver disease complications were more than tripled in the cohort of patients with cirrhosis than in the cohort without cirrhosis. Among the 4 regimens, SOF+PR had the most favorable health outcomes.......Sustained virologic response (SVR) and adverse event rates were based on published data (SVR = 92% vs. 80% for SOF+PR, 75% vs. 62% for TVR+PR, 64% vs. 55% for BOC+PR, and 58% vs. 33% for PR in patients without and with cirrhosis, respectively)"
Reported by Jules Levin
Presented at AASLD 3013
Program abstract
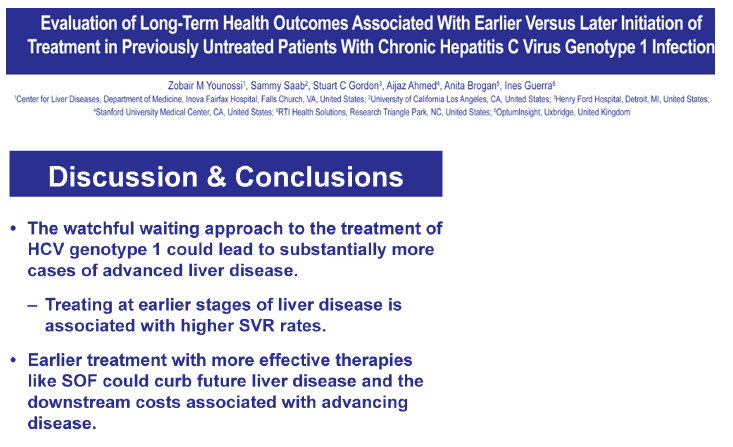
BACKGROUND & AIM: To address the ongoing debate on the downstream costs and sequelae associated with waiting to treat chronically infected hepatitis C virus (HCV) patients, a decision analytic Markov model assessed the long-term health outcomes associated with treating patients in the non-cirrhotic stage and in the cirrhotic stage.
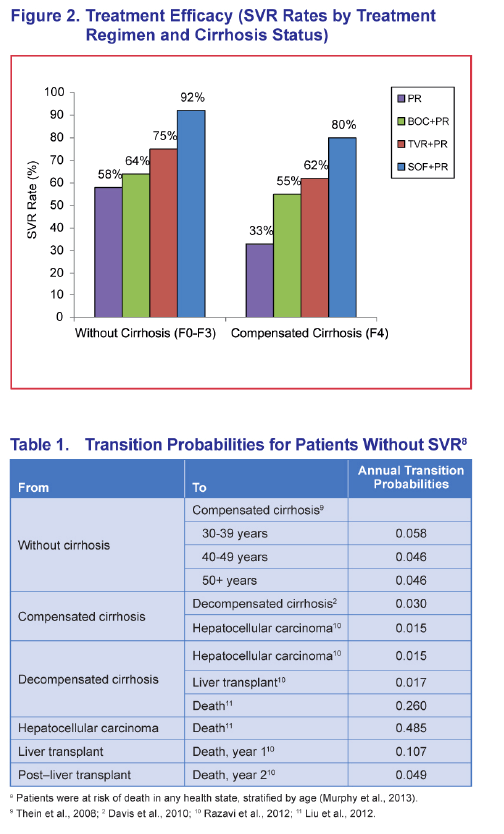
METHODS: The analysis modeled two cohorts of treatment-naïve chronic HCV genotype 1 patients with a mean age of 52 from a US third party payer perspective for a lifetime horizon: one cohort initiating treatment in the non-cirrhotic stage, and the other starting treatment in the cirrhotic stage. The model included the following regimens: sofosbuvir (SOF) in combination with pegylated interferon plus ribavirin (PR) for 12 weeks, telaprevir (TVR) plus PR for 24-48 weeks, boceprevir (BOC) plus PR for 28-48 weeks, and PR for 48 weeks. Sustained virologic response (SVR) and adverse event rates were based on published data (SVR = 92% vs. 80% for SOF+PR, 75% vs. 62% for TVR+PR, 64% vs. 55% for BOC+PR, and 58% vs. 33% for PR in patients without and with cirrhosis, respectively). Transition probability, utility, and cost estimates (in 2013 US dollars) were based on a literature review, public sources, and consensus by a panel of 4 hepatologists.
RESULTS: Cases of liver disease complications were more than tripled in the cohort of patients with cirrhosis than in the cohort without cirrhosis. Among the 4 regimens, SOF+PR had the most favorable health outcomes.
CONCLUSIONS: This study projects that the watchful waiting approach to the treatment of HCV genotype 1 could lead to substantially more cases of advanced liver disease. Earlier treatment with more effective therapies like sofosbuvir could curb future liver disease and the downstream costs associated with advancing disease.



|
|
| |
| |
|
|
|