| |
Costs of telaprevir-based triple therapy for hepatitis C: $189,000 per sustained virological response
|
| |
| |
Download the PDF here
Hepatology Oct 2014
Kian Bichoupan,1 Valerie Martel-Laferriere,1 David Sachs,2 Michel Ng,1 Emily A. Schonfeld,1 Alexis Pappas,1 James Crismale,1 Alicia Stivala,1 Viktoriya Khaitova,1 Donald Gardenier,1 Michael Linderman,2 Ponni V. Perumalswami,1 Thomas D. Schiano,1 Joseph A. Odin,1 Lawrence Liu,1 Alan J. Moskowitz,3 Douglas T. Dieterich,1 and Andrea D. Branch1
"In summary, our analysis of TVR-based triple therapy in real-world practice showed that this intervention is less effective and more costly than projected. The SVR rate was 44%, and the cost per SVR was almost $190,000. Our study holds important information for other countries continuing to use TVR.[33, 34] This study provides data that will be valuable for future cost comparisons and highlights the importance of investigating new regimens outside formal clinical trials." Look at subgroup analyses for costs, particularly gt1a, blacks, advanced disease
ABSTRACT-
In registration trials, triple therapy with telaprevir (TVR), pegylated interferon (Peg-IFN), and ribavirin (RBV) achieved sustained virological response (SVR) rates between 64% and 75%, but the clinical effectiveness and economic burdens of this treatment in real-world practice remain to be determined. Records of 147 patients who initiated TVR-based triple therapy at the Mount Sinai Medical Center (May-December 2011) were reviewed. Direct medical costs for pretreatment, on-treatment, and posttreatment care were calculated using data from Medicare reimbursement databases, RED Book, and the Healthcare Cost and Utilization Project database. Costs are presented in 2012 U.S. dollars. SVR (undetectable hepatitis C virus [HCV] RNA 24 weeks after the end of treatment) was determined on an intention-to-treat basis. Cost per SVR was calculated by dividing the median cost by the SVR rate. Median age of the 147 patients was 56 years (interquartile range [IQR] = 51-61), 68% were male, 19% were black, 11% had human immunodeficiency virus/HCV coinfection, 36% had advanced fibrosis/cirrhosis (FIB-4 scores ≥3.25), and 44% achieved an SVR. The total cost of care was $11.56 million. Median cost of care was $83,721 per patient (IQR = $66,652-$98,102). The median cost per SVR was $189,338 (IQR = $150,735-$221,860). Total costs were TVR (61%), IFN (24%), RBV (4%), adverse event management (8%), professional fees (2%), and laboratory tests (1%). Conclusions: TVR and Peg-IFN accounted for 85% of costs. Pharmaceutical prices and the low (44%) SVR rate, in this real-world study, were major contributors to the high cost per SVR.
Abbreviations
AEs adverse events
EOT end of treatment
EPO erythropoetin-α
ER emergency room
HCC hepatocellular carcinoma
HCV hepatitis C virus
HIV human immunodeficiency virus
ICD-9 International Classification of Diseases, Ninth Revision
IQR interquartile range
ITT intention to treat
LLOD lower limit of detection
Peg-IFN pegylated interferon
RBV ribavirin
RGT response-guided therapy
SIM simeprevir
SOF sofosbuvir
SVR sustained virological response
TVR telaprevir
VF virological failure
WAC wholesale acquisition cost
Hepatitis C virus (HCV) is a major public health threat. There are approximately 180 million HCV-infected people worldwide, and the estimated number in the United States ranges from 2.7 to 4 million.[1-4] HCV infection causes a slowly progressive disease in most patients and can lead to liver cirrhosis, hepatocellular carcinoma (HCC), liver failure, and death.[5] The average age of the HCV-infected population is increasing and the extent of liver disease is increasing along with it, intensifying the urgency of finding and implementing effective treatments. By 2030, 45% of the HCV-infected persons in the United States are projected to have liver cirrhosis.[6] A recent study raises concern that mortality among HCV-infected persons may be increasing.[4]
It is important to identify the most clinically and cost-effective strategy for reducing the burden of HCV-related liver disease. HCV-positive patients have higher health care costs than HCV-negative patients.[7-9] Costs increase as liver disease worsens.[10] Estimated mean annual health-care-related costs are approximately $17,000 for patients without liver cirrhosis and $60,000 for those with end-stage liver disease.[10] Without dramatic changes in disease management, total health care costs are projected to peak in 2024 at $9.1 billion, with treatment of decompensated cirrhosis accounting for 46%.[11]
The aim of HCV treatment is to interrupt disease progression and potentially allow some repair to occur. Successful treatment results in a sustained virological response (SVR), which has historically been defined as the absence of HCV viral RNA 24 weeks after the end of treatment (EOT). Patients who achieve an SVR have lower rates of all-cause mortality, liver-related mortality, liver decompensation, HCC, and cirrhosis than patients who are nonresponders.[12-16] These benefits have been demonstrated most clearly for patients with advanced disease[18]; however, because patients with liver cirrhosis who achieve an SVR remain at elevated risk for the development of HCC, Koh et al. have postulated that the greatest benefit from SVR may be in patients without cirrhosis.[17] These findings strongly suggest that an SVR improves health and reduces health care costs. However, the magnitude of the savings is uncertain and dependent on factors that are changing over time, such as the health status of the HCV-positive population and the clinical effectiveness and cost of antiviral therapy. The surprising similarity of long-term clinical outcomes of patients who relapse after achieving an EOT and patients who remain HCV viral load undetectable contributes to the uncertainty[18, 19] and underscores the need for information about the clinical and economic significance of treatment.
Before May 2011, the standard treatment for genotype 1 HCV was 48 weeks of dual therapy with pegylated interferon (Peg-IFN) and ribavirin (RBV). This treatment had SVR rates of 35%-45% in phase III clinical trials[20, 21] at a cost of approximately $70,364 per SVR.[22] In 2011, telaprevir (TVR), a first-generation direct-acting antiviral drug targeting the HCV nonstructural 3/4A protease, received U.S. Food and Drug Administration approval for use in genotype 1 HCV in combination with IFN/RBV (triple therapy). Triple therapy achieved SVR rates between 64% and 75% in the phase III clinical trials.[23-25] At these success rates, HCV triple therapy was considered cost-effective[26-30]; however, real-world data about SVR rates and adverse events (AEs) are needed to reach final conclusions. Severe AEs[23-25] and several deaths have been reported,[31, 32] raising safety concerns. The phase III trials enrolled relatively few blacks, patients with advanced fibrosis or cirrhosis, or patients above the age of 65 years,[23-25] yet many people in these groups need care and wish to be treated. Despite the approval of newer agents in the United States and Europe, TVR remains the standard of care in other countries around the world, such as Australia.[34]
This investigation addresses the need for additional information about outcomes and costs of TVR-based triple therapy. To our knowledge, this is the first report of outcomes and direct medical costs of this regimen in real-world practice.
Materials and Methods
Study Design and Patients
Study subjects were identified using a combination of traditional and enhanced information technology methods. In one case-finding method, health care providers at the Mount Sinai Medical Center (New York, NY) compiled lists of patients with chronic genotype 1 HCV infection who initiated TVR-based triple therapy between May and December 2011. In the other, patients were identified by querying the Mount Sinai Data Warehouse, a database that integrates multiple electronic health record platforms. The automated process generated a list of patients whose record included the International Classification of Diseases, Ninth Revision (ICD-9), code 070.54 and a TVR prescription between May and December 2011. The lists generated by the two methods were inspected and disparities were resolved by examining medical records, yielding a cohort of 147 case patients. The combination of these two methods ensured that all patients receiving at least one dose of TVR were included. Patients with a previous liver transplant were excluded. Most patients received standard TVR-based triple therapy, with 750 mg of TVR three times a day for 12 weeks and IFN and weight-based RBV for 48 weeks. Because of a known drug-drug interaction, human immunodeficiency virus (HIV)/HCV coinfected patients on efavirenz received 1,125 mg TVR three times a day. Patients were eligible 24 weeks of response-guided therapy (RGT) if they were without cirrhosis, treatment naïve, or relapsers to dual therapy, HCV monoinfected, and had an undetectable HCV viral load at weeks 4 and 12. No patient was treated for more than 48 weeks. AEs were managed by health care providers according to clinical judgment. Generally, the dose of RBV was reduced when hemoglobin dropped below 10 g/dL and, simultaneously, a request for authorization of erythropoeitin-α (EPO) use was submitted. However, some patients received EPO preceding a RBV dose reduction.
Data on demographics, HCV kinetics, clinical laboratory tests, office visits, medications, AE management, and other aspects of medical care were collected at baseline and other key time points, typically at weeks 4, 12, 24, and 48 during treatment and at weeks 12 and 24 posttreatment. Outcomes of earlier dual therapy were extracted from laboratory reports and clinical notes and coded as follows: Patients with undetectable HCV RNA at the EOT who later had detectable HCV RNA were coded as "relapsers"; those whose HCV RNA remained detectable throughout treatment were coded as "nonresponders"; and those who were not able to complete previous therapy because of the AEs and side effects were coded as "intolerant."
HCV viral load was measured using a real-time polymerase chain reaction assay (Cobas Ampliprep Cobas Taqman version 2.0; Roche Molecular Diagnostics, Pleasanton, CA). HCV viral load below the lower limit of detection (LLOD; 18 IU/mL) was coded as "undetectable." Virological failure (VF) was defined as a viral load >1,000 IU/mL between weeks 4 and 24 or above the LLOD after week 24. The FIB-4 score was used to estimate the extent of liver fibrosis,[35-37] with a value ≥3.25 indicating advanced fibrosis/cirrhosis. The SVR rate was determined on an intention-to-treat (ITT) basis. Undetectable HCV RNA was imputed for missing time points if HCV RNA was undetectable before and after. The study was conducted in accord with the Helsinki agreement, with approval of the Mount Sinai Institutional Review Board (GCO10-0032).
Use of Resources and Costs
Pretreatment costs included clinical laboratory tests, imaging, and office visits. On-treatment costs included HCV medications, AE management, clinical laboratory tests, and office visits. Posttreatment costs included clinical laboratory tests, posttreatment AE management, and office visits.
Supporting Table 1 lists the costs of HCV medications, AE pharmaceuticals and biologics, hospitalizations, emergency room (ER) visits, office visits, and clinical laboratory tests. The wholesale acquisition costs (WACs) of HCV medications were obtained from the Red Book in 2012. Hospitalizations and ER visits were classified by ICD-9 codes, which were used to estimate charges based on the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (2010) and the Nationwide Emergency Department Sample (2008). Hospitalizations charges were converted to costs by multiplying charges by 0.38, which was the nation-wide average cost-to-charge ratio for the hospitalizations observed.[36, 38] ER costs were approximated by multiplying ER charges by 0.27, which was the nation-wide average Medicare payment-to-charge ratio.[36, 38] Cost of care included HCV medications (TVR, IFN, and RBV), AE management, and professional fees, as well as clinical laboratory tests. All costs were expressed in 2012 U.S. dollars. Cost per SVR was calculated by dividing the median cost by the SVR rate.
Sensitivity Analysis
Univariable sensitivity analyses were conducted to determine the impact of the SVR rate and TVR and IFN prices on the cost per SVR. The rate of SVR was varied over a range of 20%-75%, and the TVR and IFN prices were varied over a range of 80%-120% of the WAC. To assess the impact of time of treatment initiation on SVR, we evenly divided the cohort into two groups and analyzed them separately for SVR rates and cost of care.
Statistical Analysis
Costs are presented as the median and interquartile range (IQR). In univariable analysis, t tests were used for normally distributed continuous variables and Mann-Whitney's U tests for non-normally distributed variables. Chi-square or Fisher's exact tests were used for categorical variables. A P value below 0.05 was considered significant. SPSS software (version 22; SPSS, Inc., Chicago, IL) was used for statistical analysis.
Results
Baseline Characteristics of the 147 Patients on TVR-Based Triple Therapy
Table 1 shows the characteristics for the study group at baseline. The median age was 56 years (IQR, 51-61), 100 (68%) were male, 28 (19%) were black, and 16 (11%) were HIV positive. The median FIB-4 score was 2.52 (IQR, 1.77-4.26): 35% of the patients had a score ≥3.25, indicating advanced fibrosis/cirrhosis (Metavir F3-F4).[35] The majority (73%) had received dual therapy in the past: 68 (46%) were nonresponders, 29 (20%) were relapsers, and 10 (7%) were IFN intolerant.
Outcomes
Sixty-five (44%) patients achieved an SVR. Sixty-nine (47%) patients stopped treatment before completing the planned regimen, and the majority (87%) failed treatment. Twenty-two patients (15%) stopped during the first 12 weeks. Among the 82 (56%) patients who did not achieve an SVR, 42 had an inadequate virological response and terminated treatment according to stopping rules, 15 discontinued early because AEs, 18 relapsed after the EOT, and 7 were lost to follow-up. Figure 1 shows the SVR rates of patients in various subgroups: those who experienced VF (0 of 45; 0%); discontinued because of AEs (7 of 22; 32%); completed RGT (9 of 15; 60%); completed 48 weeks of treatment (47 of 63; 75%); and discontinued because of social reasons (2 of 2; 100%). Figure 2 shows the times during treatment when patients had confirmed evidence of treatment failure separated into those who discontinued because of VF or AEs.
A comparison of SVR rates between various subgroups can be seen in Supporting Table 2. The SVR rate was higher in whites than in blacks (50% vs. 21%; P < 0.01) and was higher in patients who completed the planned treatment than in those who discontinued treatment early because of AEs or for social reasons (72% vs. 38%; P < 0.01). SVR rates did not differ significantly by gender, previous treatment response, completion of standard treatment or RGT, HIV/HCV coinfection, and FIB-4 score below and above 3.25.
Cost of Care
The total cost of care for all 147 patients was $11.56 million. Of this, the SVR group (n = 65) accounted for $6.32 million (55%) and the non-SVR group (n = 82) accounted for $5.24 million (45%). The median cost of care per patient was $83,721 (Table 2). Of the subgroups analyzed, the cost per patient was highest for patients completing 48 weeks of treatment ($99,357) and lowest for patients who discontinued treatment early because of AEs ($51,778). The total costs for HCV medications, AE management, fees for professional services, and clinical laboratory tests are listed in Table 3.
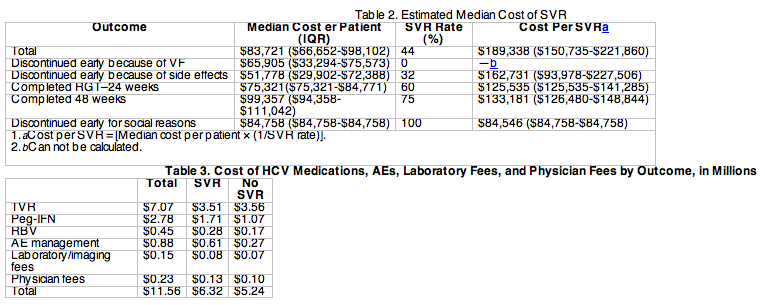
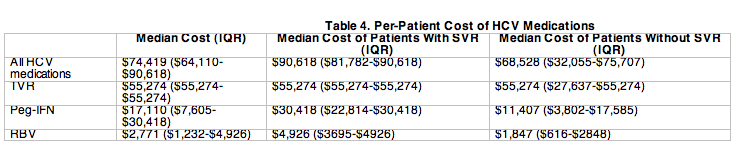
HCV medications were the largest component of costs, totaling $10.30 million for the 147 patients. Table 4 shows the per-patient cost of HCV medications. The median cost per patient of these medications was $74,419, of which $55,274 was for TVR, $17,110 was for IFN, and $2,771 was for RBV. TVR, IFN, and RBV accounted for 69%, 27%, and 4% of medication costs, respectively.
AE management costs totaled $0.88 million, 8% of the total cost of care.
Eighty-three patients (56% of the cohort) had costs for AE management, which included medications/biologics and blood, hospitalizations, and ER visits. These costs were higher in the SVR group (total, $611,049; median, $10,500) than in the non-SVR group (total, $266,561; median, $4,829; P < 0.01), as expected because of the longer duration of treatment in the SVR group and accompanying AEs. Supporting Table 3 shows the cost of medications and blood transfusions for AE management. Seventy-one patients received EPO, at a total cost of $701,893, 14 received filgrastim, at a total cost of $32,704, and 13 had blood transfusions, at a total cost of $13,066. Supporting Tables 4 and 5 show the costs of ER visits and hospitalizations grouped by ICD-9 code. ER costs totaled $9,214, with 27% used to treat anemia. Hospitalization costs totaled $102,403, with 54% used to treat anemia, 14% to treat infections, and 14% to treat renal insufficiency. Professional fees accounted for $234,357 (2% total costs). Clinical laboratory tests and imaging accounted for $147,740 (1% of total costs).
Cost Per SVR and Sensitivity Analyses
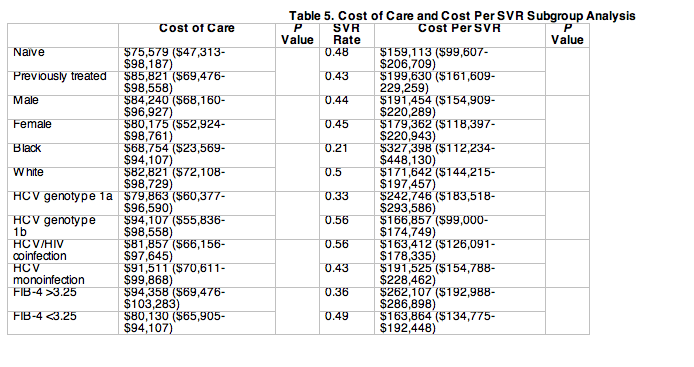
The low (44%) SVR rate drove the cost per SVR to $189,338. The cost per SVR was higher in groups with lower SVR rates (Table 2). The cost per SVR was examined in various subgroups (Table 5). It was higher in previously treated patients than treatment-naïve patients (P < 0.01), in blacks than in nonblacks (P < 0.01), in patients with genotype 1a HCV than in those with genotype 1b HCV (P < 0.01), in patients with HCV monoinfection than in those with HIV/HCV coinfection (P = 0.02), and in patients with a FIB-4 score ≥3.25 than in those with FIB-4 <3.25 (P < 0.01). Cost per SVR could not be calculated for relapsers because all of them failed therapy. However, it is important to keep in mind that relapsers contributed disproportionately to the median cost per SVR of the study group, underscoring the importance of preventing relapse.
In one-way sensitivity analyses, the median cost per SVR ranged from $418,059 to $111,482 across SVR rates of 20%-75%, keeping costs of medications constant. The cost per SVR ranged from $164,090 to $214,092 across TVR prices ±20% of the WAC price at an SVR rate of 44% and ranged from $181,118 to $195,970 across IFN prices ±20% of the WAC at an SVR rate of 44%.[39]
To determine whether outcomes improved as providers gained experience with TVR, data on patients initiating treatment during the first half of the study were compared to those of patients initiating treatment during the second half; however, no significant differences were found for SVR rates (42% vs. 47%; P = 0.57), duration of treatment (25 weeks vs. 30 weeks; P = 0.32), or cost per SVR ($190,151 vs. $184,076; P = 0.28). There was a nonsignificant trend in the percentage of patients completing therapy (from 45% and 60%; P = 0.08).
Discussion
This study reports the first data about the relationship between the clinical effectiveness and costs of TVR-based triple therapy in real-world clinical practice. Our four major findings were (1) the SVR rate was 44%, (2) the median cost per SVR was $189,338, (3) TVR and IFN were the most important components of costs, accounting for approximately 85% of the total, and (4) 56% of patients had AEs that required management. Including HCV medications, AE costs, professional fees, and clinical laboratory tests, the median cost of care per patient was $83,721. The high cost per SVR was driven by the costs of TVR and IFN and the low effectiveness of treatment. VF and side effects caused early discontinuation in many patients, with only 53% completing therapy.
The cost per SVR in this study was more than double the projection by Thorlund et al.,[40] who estimated a cost per SVR for TVR-based therapy of $74,380-$76,370 for previously treated and untreated patients. Their projection was based on data from clinical trials and used an SVR rate between 70% and 90%. The discontinuation rate in our cohort was higher and the SVR rate was lower than the values they used, accounting for the disparity.
Effectiveness in clinical practice is typically lower than the efficacy achieved under the tightly controlled conditions of a trial, with the difference attributed to several factors, including the inclusion of a broader range of patients, older patients, patients with complex medical conditions, and patients who may be less adherent to treatment and other health-promoting practices than those in clinical trials. Our data enable the development of models based on real-world experience with a cohort of patients closely resembling the HCV-positive population in the United States. Within the U.S. population with chronic HCV, 39.5% are estimated to have Metavir F3-F4 fibrosis,[5] 22% are black,[41] and 5% are over the age of 65.[42] In our cohort, 35% had F3-F4 fibrosis (based on the FIB-4 score), 19% were black, 8% were over the age of 65, and 11% had HCV/HIV coinfection. In contrast, in clinical trials, 31% of patients had advanced fibrosis/cirrhosis, 9% were black, and none were HCV/HIV coinfected.[23-25]
The cost per SVR in our study was strongly affected by the cost of TVR and the SVR rate. This is consistent with two separate studies investigating the cost-effectiveness of TVR in naïve and previously treated patients conducted by Camma et al.[26, 39] They found that the incremental cost-effectiveness ratio of TVR triple therapy versus dual therapy was highly sensitive to the cost of TVR and the likelihood of SVR.[26, 29] Other studies project that all-oral therapies for HCV will be cost-effective, compared to current triple-therapy regimens.[43, 44]
Two new agents, simeprevir (SIM) and sofosbuvir (SOF), were recently approved for HCV therapy. The pharmaceutical cost of SIM and SOF are greater than TVR and boceprevir; however, costs per SVR are expected to be lower, primarily as a result of higher SVR rates. Supporting Table 6 shows the expected cost of medications and the expected cost per SVR using each regimen. In comparison to the cost of TVR-based triple therapy, which ranges from $72,946 to $90,618, the costs of currently approved multidrug regimens that contain SIM or SOF are substantially higher. The costs of these new regimens range from $84,024 to $170,472. At $150,000 per SVR, $480 billion will be required to induce an SVR in the estimated 3.2 million people in the United States who have chronic HCV infection. This is 3% of the annual gross domestic product.
To fully assess the cost-benefit ratio of various regimens, it will be important to gain a better understanding of the long-term impact on health and health care costs conferred by an SVR. A recent study provides a useful starting point. Manos et al. compared health care utilization costs before and after HCV IFN/RBV dual therapy and found that the adjusted difference in annual total mean costs between the SVR and non-SVR groups was $2,648 (95% confidence interval: $737-$4,560) over a 5-year period. More-effective therapies are expected to yield greater economic savings. IFN/RBV dual therapy selects for patients with specific baseline characteristics. On average, patients who achieve an SVR are younger and in better health than those who do not.[45] They are less likely to be black, to have HIV coinfection, and they are more likely to have a favorable ILB28B genotype.[46-49] Next-generation therapies are expected to allow nearly all patients to achieve an SVR, not a selected subset. When this occurs, the economic benefits of SVR may be much greater than reported by Manos et al. Treatment will be especially beneficial if SVR leads to a long-term reduction in one or more of the comorbid conditions that are prevalent in the HCV-positive population.[50]
Our study has several strengths and some limitations. An independent group conducted a similar study and had identical results; the cost per patient was $83,376 and the cost per SVR was $183,428.[51] Our SVR rate, which was lower than observed in the clinical trials, has been reported in other studies, such as the CUPIC and TARGET.[52, 53] As mentioned above, the cohort was racially diverse and included patients with a spectrum of liver disease and a wide range of ages. Cost estimates were based on events recorded in the medical record, rather than on group averages, which are often used to estimate health care utilization costs. However, AEs may have been under-reported in the medical record, leading to an underestimate of AE-associated costs. Treatment costs covered by the patients, such as costs of over-the-counter medications and transportation, and the personal burdens of treatment, such as reduced productivity at work and reduced quality of life, were not included and may have been substantial. WAC prices were used instead of average wholesale prices, which may have underestimated medication costs. Seven patients (5% of the cohort) were lost to follow-up, potentially causing the SVR rate to be a slight underestimate in our ITT analysis (if one or more of these patients achieved an SVR). Because cost-to-charge ratios for ER visits were not available, ER costs were approximated by multiplying charges by Medicare payment to charge ratios. Few patients were candidates for RGT and this may have increased costs. Finally, the entire cohort received TVR-based triple therapy, and we are thus unable to directly compare the cost per SVR to alternative therapies.
In summary, our analysis of TVR-based triple therapy in real-world practice showed that this intervention is less effective and more costly than projected. The SVR rate was 44%, and the cost per SVR was almost $190,000. Our study holds important information for other countries continuing to use TVR.[33, 34] This study provides data that will be valuable for future cost comparisons and highlights the importance of investigating new regimens outside formal clinical trials.
|
|
| |
| |
|
|
|