 |
 |
 |
| |
All-Oral Therapy with Sofosbuvir Plus Ribavirin for the Treatment of HCV Genotype 1, 2,3 and 4 Infection in Patients Coinfected with HIV (PHOTON-2)
|
| |
| |
Reported by Jules Levin
IAS 2014, Melbourne, Australia
Jean-Michel Molina1, Chloe Orkin2, David M. Iser3,Francisco.X. Zamora4, Mark Nelson5, Christoph Stephan6, Benedetta Massetto7, Anuj Gaggar7, Liyun Ni7, Evguenia Svarovskaia7, Diana Brainard7, G. Mani Subramanian7, John G. McHutchison7, Massimo Puoti8, Jurgen K. Rockstroh9
1University of Paris Diderot, Paris 7 and Department of Infectious Diseases, Saint-Louis Hospital, Paris, France; 2Barts Health NHS Trust, London, UK; 3Infectious Diseases Unit, Alfred Hospital, Melbourne, Australia; 4HIV Unit, Internal Medicine Service, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain; 5Chelsea and Westminster Hospital, St. Stephens Centre, London, UK; 6Infectious Diseases Unit at Medical Department, Hospital of the Johann Wolfgang Goethe-University, Frankfurt, Germany; 7Gilead Sciences, Inc., Foster City, CA, USA; 8Division of Infectious Diseases, AO Ospedale Niguarda Ca' Granda, Milan, Italy; 9Department of General Internal Medicine I, University Hospital of Bonn, Bonn, Germany
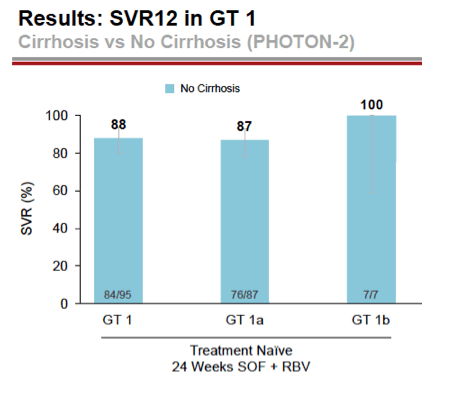
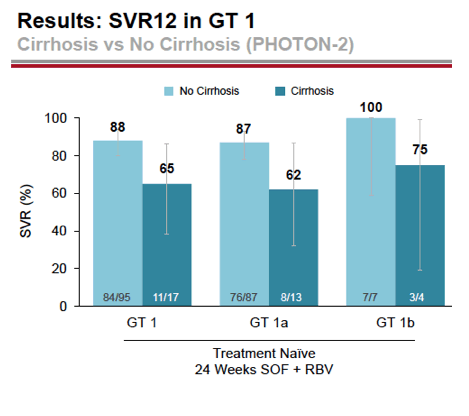
- 88% (84/95) with GT1 without cirrhosis had SVR12: 88% Gt1a (76/87), 100% Gt1b (7/7)
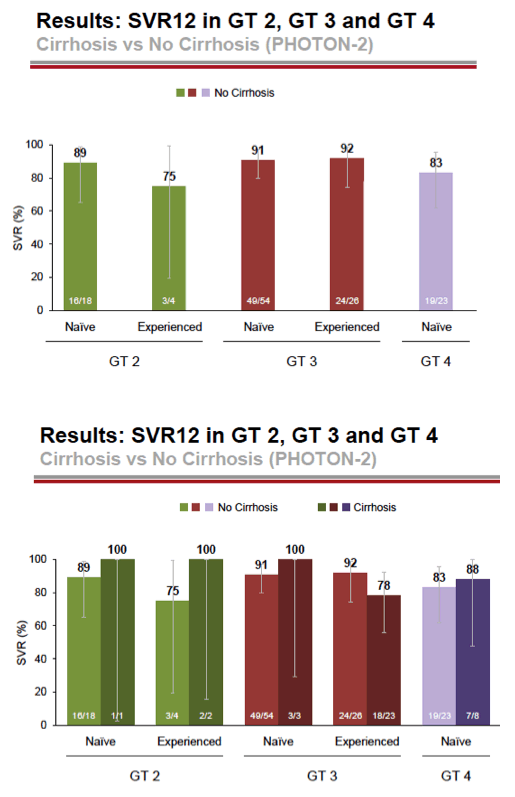
- 89% (16/18) of treatment-naive GT2 without cirrhosis had SVR12, 24 weeks treatment
- 91% (49/54) treatment-naive Gt3 without cirrhosis had SVR12, 24 weeks treatment
- 83% (19/23) GT4 treatment-naive without cirrhosis had SVR 12, 24 weeks treatment
- GT2, 89% (16/18) treatment-naive & 75% (3/4) treatment experienced without cirrhosis had SVR12, 12 weeks treatment
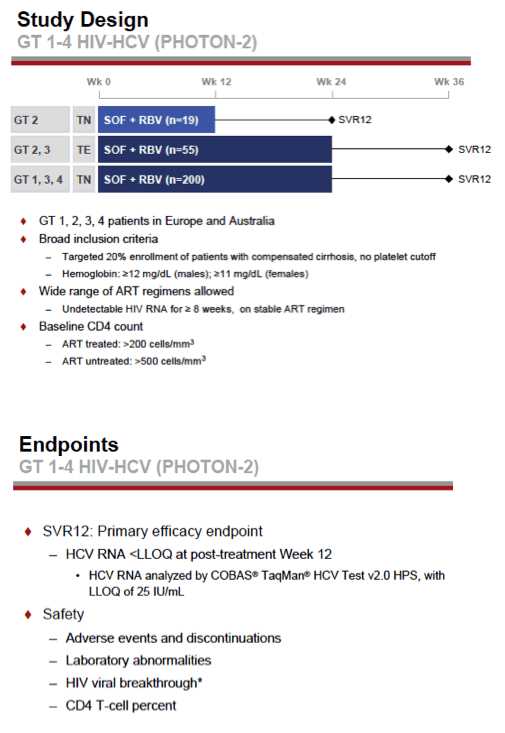
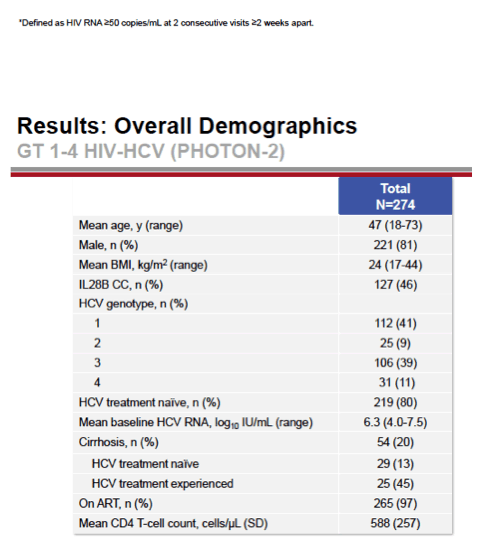
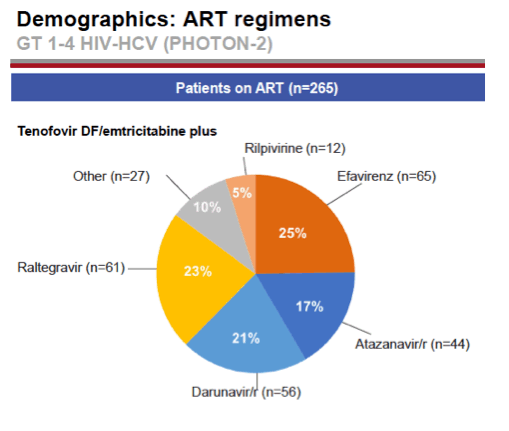
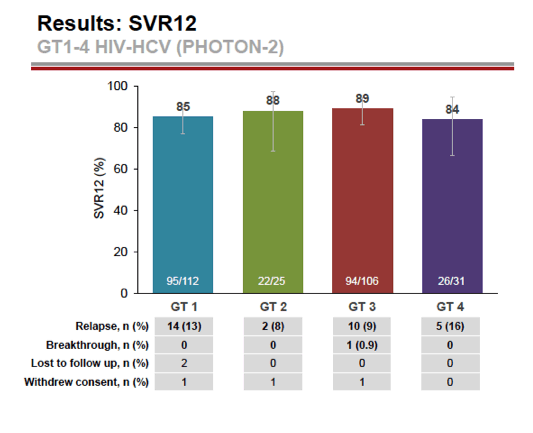
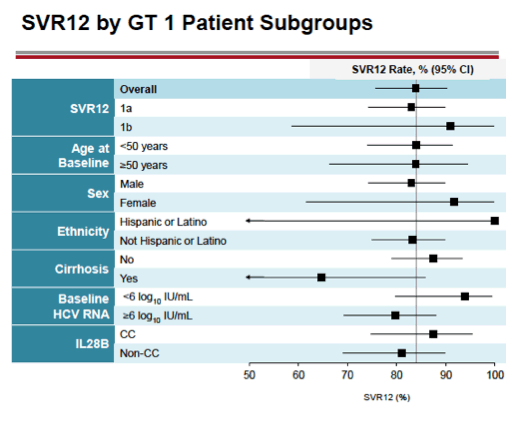
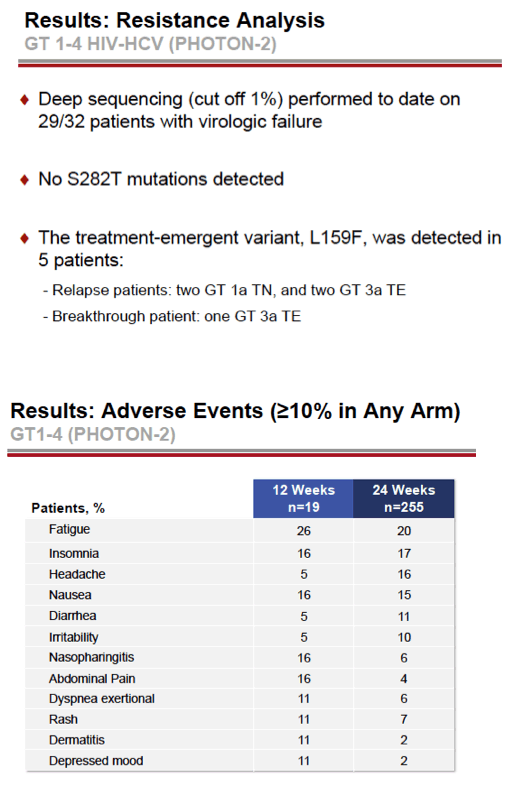
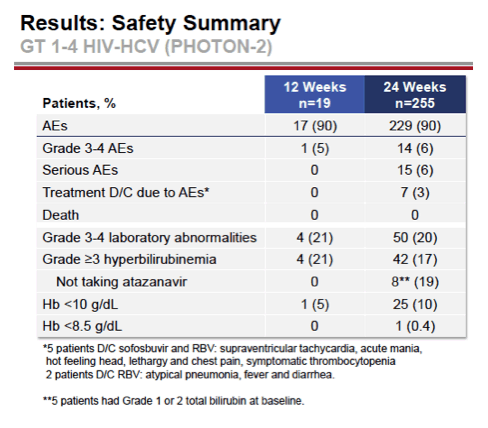
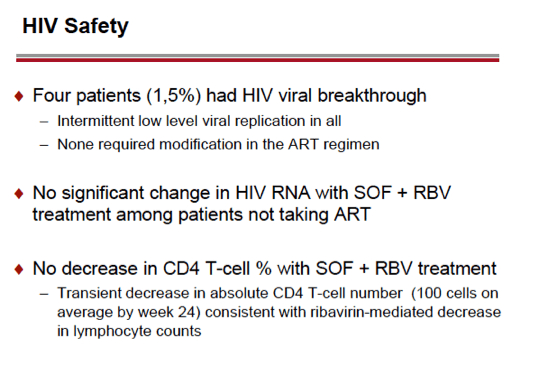
see charts below for full results

|
| |
|
 |
 |
|
|