 |
 |
 |
| |
Once-Daily Maraviroc Inferior to TDF/FTC in
First-Line Regimens With Darunavir
|
| |
| |
20th International AIDS Conference, July 20-25, 2014, Melbourne
Mark Mascolini
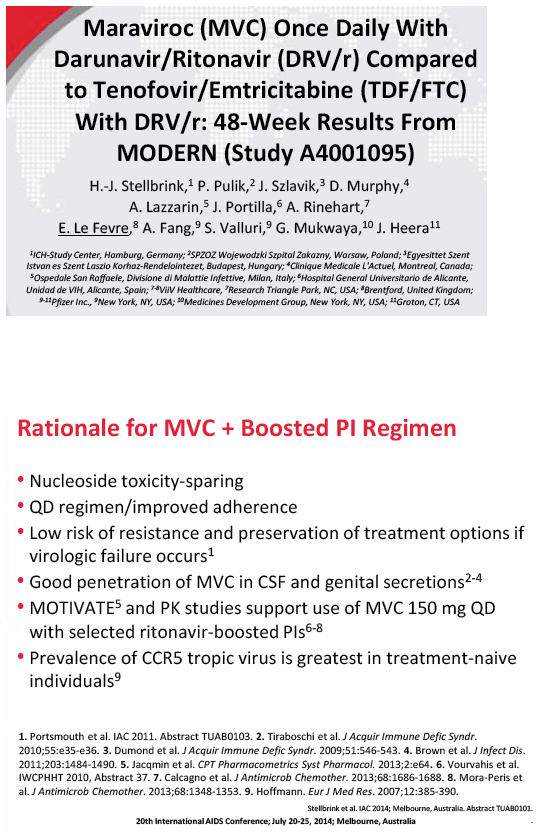
A once-daily regimen combining the CCR5 antagonist maraviroc with the protease inhibitors (PIs) darunavir/ritonavir proved inferior to tenofovir/emtricitabine (TDF/FTC) plus the PIs in an 800-person randomized double-blind trial [1]. Lack of efficacy--not side effects--accounted for the difference between the two regimens.
Nucleoside/nucleotide-sparing regimens have long seemed an alluring first-line option because such combinations may lower pill burden, toxicity, cost, and drug-drug interactions. But US antiretroviral guidelines still recommend no first-line combinations lacking two nukes, and demonstrating the efficacy of such regimens has proved difficult. A 112-person single-arm trial of darunavir/ritonavir plus the integrase inhibitor raltegravir in antiretroviral-naive people recorded virologic failure rates of 16% by week 24 and 26% by week 48--considered high by current standards [2].
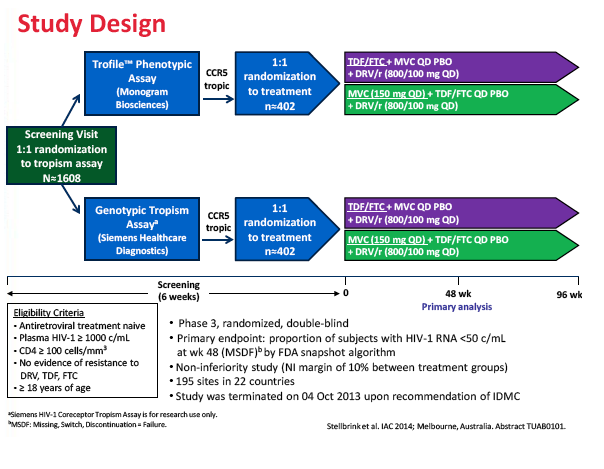
The double-blind phase 3 MODERN trial enrolled antiretroviral-naive adults with a viral load above 1000 copies and without detectable resistance to darunavir, TDF, or FTC [1,3]. The investigators randomized them to genotyping or the Enhanced Sensitivity Trofile Assay to identify people with CCR5-using HIV. Then they randomized people with CCR5-using virus to 150 mg of maraviroc once daily or 200/300 mg of TDF/FTC once daily, each with 800/100 mg of darunavir/ritonavir daily. (The licensed dose of maraviroc is 150 mg twice daily with boosted PIs except tipranavir/ritonavir, though pharmacokinetic studies suggest a once-daily dose is feasible with certain boosted PIs.) The primary endpoint was the proportion of people with a viral load below 50 copies at week 48 in an FDA snapshot analysis.
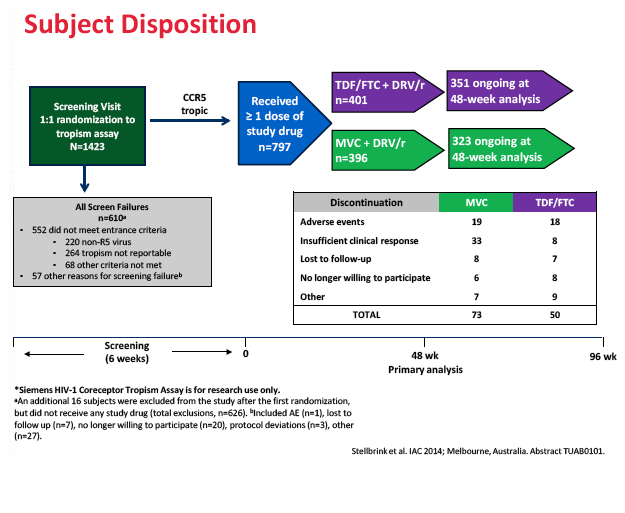
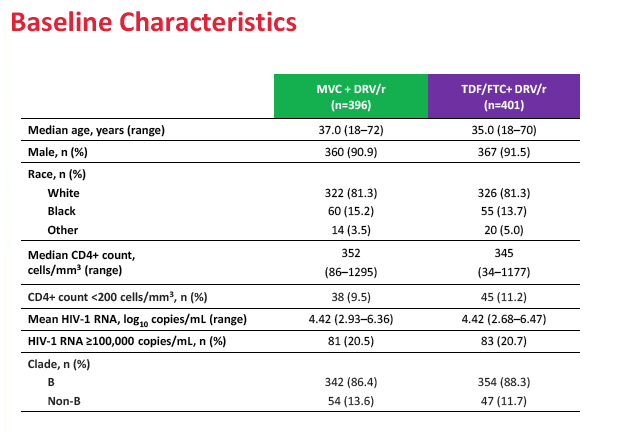
The study took place at 195 sites in 22 countries including the United States, Canada, France, Germany, Italy, the Netherlands, Spain, and the United Kingdom. Of the 797 study participants who started study drugs, 396 took maraviroc and 401 took TDF/FTC. Median pretreatment age in the maraviroc arm and the TDF/FTC arm stood at 37 and 35 years. Respective proportions of men were 90.9% and 91.5%, of whites 81.3% and 81.3%, and of blacks 15.2% and 13.7%. Median pretreatment CD4 count stood at 352 in the maraviroc group and 345 in the TDF/FTC group. Pretreatment viral load averaged 4.42 log10 copies/mL (about 26,000 copies) in both groups, and about 20% in each group had a pretreatment load above 100,000 copies.
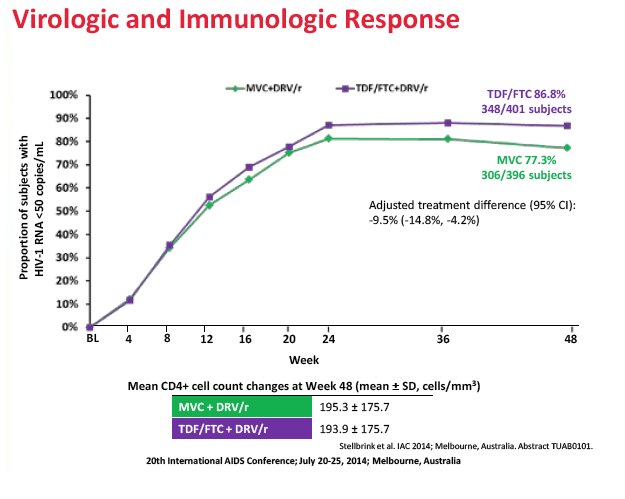
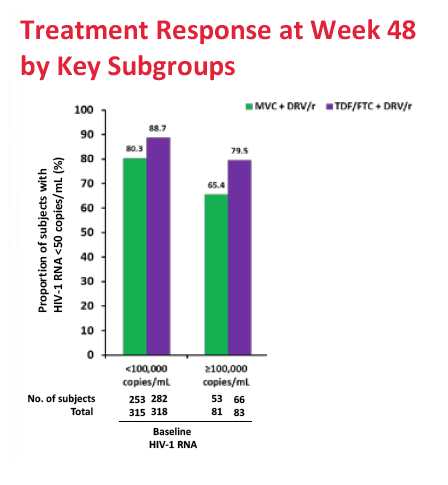
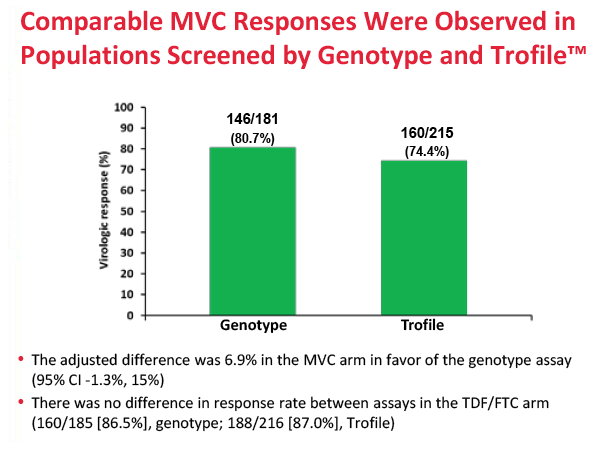
After 48 weeks of treatment, 77.3% in the maraviroc arm and 86.8% in the TDF/FTC arm had a viral load below 50 copies. The adjusted difference (-9.5%, 95% confidence interval -14.8% to -4.2%) did not meet the -10.0% noninferiority criteria, meaning maraviroc/darunavir is inferior to TDF/FTC/darunavir in an antiretroviral-naive population. Week-48 sub-50-copy response rates were 80.3% with maraviroc and 88.7% with TDF/FTC in people who began treatment with a viral load below 100,000 copies. The response difference was greater among people with a pretreatment load above 100,000 copies--65.4% with maraviroc versus 79.5% with TDF/FTC. The proportion of people who met the primary endpoint did not differ significantly according to whether genotyping or the Trofile assay was used. CD4-cell gains averaged about 195 in both treatment arms.
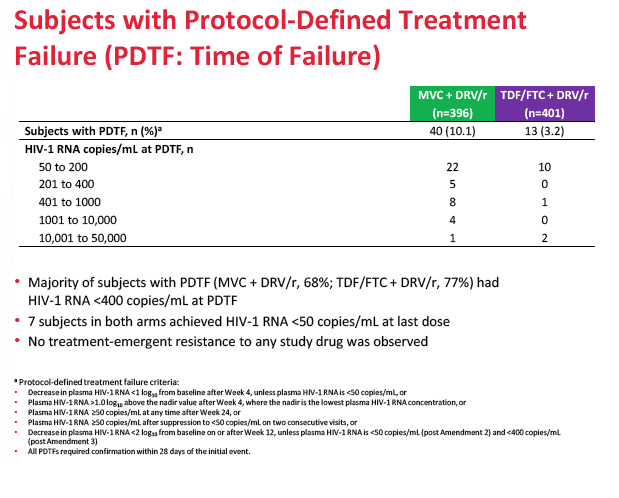
A higher proportion of people randomized to maraviroc than to TDF/FTC stopped treatment because of lack of efficacy (8.3% versus 2.0%), and a higher proportion randomized to maraviroc had protocol-defined treatment failure (10.1% versus 3.2%). Most people with protocol-defined failure (68% on maraviroc and 77% on TDF/FTC) had a viral load below 400 copies at failure. No one had treatment-emergent resistance to any drug at failure. The MODERN Data and Safety Monitoring Board recommended that the trial end early because of the inferior efficacy of the maraviroc regimen.
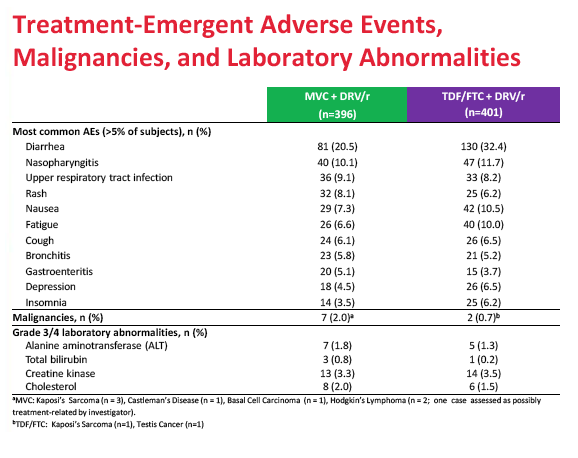
Treatment discontinuations because of adverse events did not differ substantially between the maraviroc and TDF/FTC arms (4.8% and 4.5%). Nor did the two arms differ significantly in category C (AIDS) events, grade 3 or 4 adverse events, or grade 3 or 4 lab abnormalities. The trial revealed no new or unique safety findings.
The ongoing MARCH trial is evaluating maraviroc plus a ritonavir-boosted PI as a stable switch regimen.
References
1. Stellbrink HJ, Pulik P, Szlavik J, et al. Maraviroc (MVC) dosed once daily with darunavir/ritonavir (DRV/r) in a 2 drug-regimen compared to emtricitabine/tenofovir (TDF/FTC) with DRV/r; 48-week results from MODERN (Study A4001095). AIDS 2014. 20th International AIDS Conference. July 20-25, 2014. Melbourne. Abstract TUAB0101.
2. Taiwo B, Zheng L, Gallien S, et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262). AIDS. 2011;25:2113-2122.
3. ClinicalTrials.gov. Comparative trial of maraviroc versus emtricitabine/tenofovir both with darunavir/ritonavir in antiretroviral-naive patients infected with CCR5-tropic HIV 1 (MODERN). http://clinicaltrials.gov/ct2/show/NCT01345630
4. ClinicalTrials.gov. Maraviroc Switch Collaborative Study (MARCH). http://clinicaltrials.gov/ct2/show/NCT01384682
--------------------------------------------
Maraviroc (MVC) Once Daily With Darunavir/Ritonavir(DRV/r) Compared to Tenofovir/Emtricitabine (TDF/FTC) With DRV/r: 48-Week Results From MODERN (Study A4001095)
Reported by Jules Levin
20th International AIDS Conference, July 20-25, 2014, Melbourne
H.-J. Stellbrink,1P. Pulik,2J. Szlavik,3D. Murphy,4A. Lazzarin,5J. Portilla,6A. Rinehart,7E. Le Fevre,8A. Fang,9S. Valluri,9G. Mukwaya,10J. Heera111ICH-Study Center, Hamburg, Germany; 2SPZOZ Wojewodzki Szpital Zakazny, Warsaw, Poland; 3Egyesittet Szent Istvan es Szent Laszio Korhaz-Rendelointezet, Budapest, Hungary; 4Clinique Medicale L'Actuel, Montreal, Canada; 5Ospedale San Raffaele, Divisione di Malattie Infettive, Milan, Italy; 6Hospital General Universitario de Alicante, Unidad de VIH, Alicante, Spain; 7-8ViiV Healthcare, 7Research Triangle Park, NC, USA; 8Brentford, United Kingdom; 9-11Pfizer Inc., 9New York, NY, USA; 10Medicines Development Group, New York, NY, USA; 11Groton, CT, USA
---------------------------
Program Abstract
Background: Maraviroc, a CCR5 receptor antagonist, has shown durable antiviral response with a favorable safety profile. Nucleos(t)ide-sparing, 2-drug regimens containing ritonavir-boosted protease inhibitors have not been extensively studied in antiretroviral-naïve subjects. Such regimens may decrease pill burden, toxicities, costs and drug-drug interactions.
Methods: In this multicenter, double-blind, Phase III study, HIV-1 infected antiretroviral-naïve adults with HIV-1 RNA >1000 copies/mL, without reported viral resistance, underwent 2-stage randomization. At screening, subjects were first randomized 1:1 to either genotype (Siemens) or Enhanced Sensitivity Trofile Assay (ESTA, Monogram BioSciences) to identify CCR5-tropic HIV-1 infection. Subjects with CCR5-tropic HIV-1 were then randomized 1:1 to receive MVC 150 mg QD or TDF/FTC 200/300 mg QD each with DRV/r 800/100 mg QD for up to 96 weeks. The primary endpoint was proportion of subjects with HIV-1 RNA < 50 copies/mL at Week 48 (FDA "snapshot" algorithm).
Results: 797 subjects were dosed (MVC n=396, TDF/FTC n=401). At baseline: median age 37.1 years, 8.8% female, 18.7% non-white, 20.6% HIV-1 RNA >100,000 copies/mL. Proportion of subjects meeting the primary endpoint was 77.3% for MVC and 87.0% for TDF/FTC (difference -9.73%; 95% CI; -15.0% to -4.4%). MVC did not meet the -10% non-inferiority criteria. The difference in the proportion of subjects meeting the primary endpoint between the tropism assays (genotype or ESTA) was not statistically significant (MVC: difference 6.86% in favor of genotyping, 95% CI -1.28% to 15.0%; TDF/FTC: difference 0.3%, 95% CI: -6.4% to 6.9%). More MVC subjects discontinued for lack of efficacy (8.3% vs 2.0%). Protocol defined treatment failure (PDTFs) were 10.1% for MVC and 3.2% for TDF/FTC.
Discontinuations due to AEs were 4.8% for MVC and 4.5% for TDF/FTC. Category C events, grade 3/4 AEs and laboratory abnormalities were similar between the treatment arms. This study was prematurely stopped for inferior efficacy following a recommendation by the data monitoring committee.
Conclusions: At Week 48, MVC dosed once daily with DRV/r in a 2 drug-regimen showed inferior efficacy to TDF/FTC + DRV/r in antiretroviral-naïve subjects. The two CCR5-tropism assays (genotype or ESTA) were similar in predicting a positive treatment outcome. There were no new or unique safety findings.
--------------------------
|
| |
|
 |
 |
|
|