 |
 |
 |
| |
Efavirenz Not Tied to Neurocognitive Impairment in Large Single-Center Study
|
| |
| |
20th International AIDS Conference, July 20-25, 2014, Melbourne
Mark Mascolini
People taking efavirenz ran no greater risk of neurocognitive impairment (NCI) than people taking nonefavirenz regimens, according to results of a single-center cross-sectional study at the National Institute for Infectious Disease (NIID) in Rome [1]. But it is possible that confounding by indication may color the results, if clinicians steered patients with neurocognitive risks away from efavirenz.
Neuropsychiatric symptoms are well-appreciated side effects of efavirenz. These symptoms may amount to more than a transient annoyance to patients. Recent analysis of four randomized AIDS Clinical Trials Group (ACTG) studies linked first-line efavirenz to a doubled risk of suicidality (suicidal ideation, suicide attempt, or completed suicide) [2]. The ACTG team advised that "an increased risk for suicidality should be considered when choosing efavirenz as part of an initial antiretroviral regimen." A randomized ACTG trial found no link between efavirenz and worse neuropsychological performance, although people randomized to efavirenz had more bad dreams and higher anxiety scores (but not higher depression scores) [3].
Yet whether efavirenz leads to NCI--as measured by standard tests--remains controversial. To address this question, NIID investigators conducted this retrospective cross-sectional study of adults taking combination antiretroviral therapy (cART) with or without efavirenz from 2000 through 2013 [1]. All study participants completed a standardized battery of 15 tests in five different neuropsychological domains.
The investigators calculated a Global NPZ-8 Deficit Score and Z scores for each cognitive domain. They classified cohort members as having NCI if they scored more than 1 standard deviation below the normal mean on at least two tests or 2 or more standard deviations below the mean on one test. The researchers also determined which patients had HIV-associated neurocognitive disorder (HAND) according to standard Frascati criteria. The analyses controlled for depression and other psychiatric disorders as potential confounders.
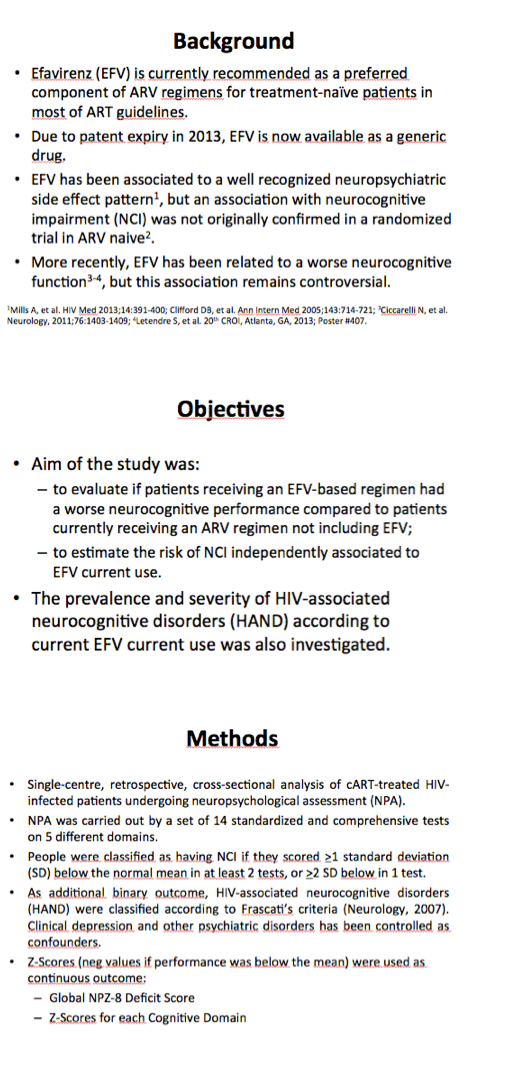
The study involved 859 antiretroviral-treated people who had 1020 neuropsychological assessments. While 732 people contributed only one neuropsychological assessment, 100 contributed two and 27 contributed three or more. Median age stood at 46 years (interquartile range 40 to 53). Three quarters of study participants (78%) were men and 28% were positive for HCV antibody. Whereas 18% acquired HIV while injecting drugs, 39% picked up HIV during sex between men and 39% during heterosexual sex. The group had a median 13 years of education. Median nadir CD4 count measured 190 and median current count 483. Two thirds of the group (69%) had a viral load below 40 copies at the time of neuropsychological testing.
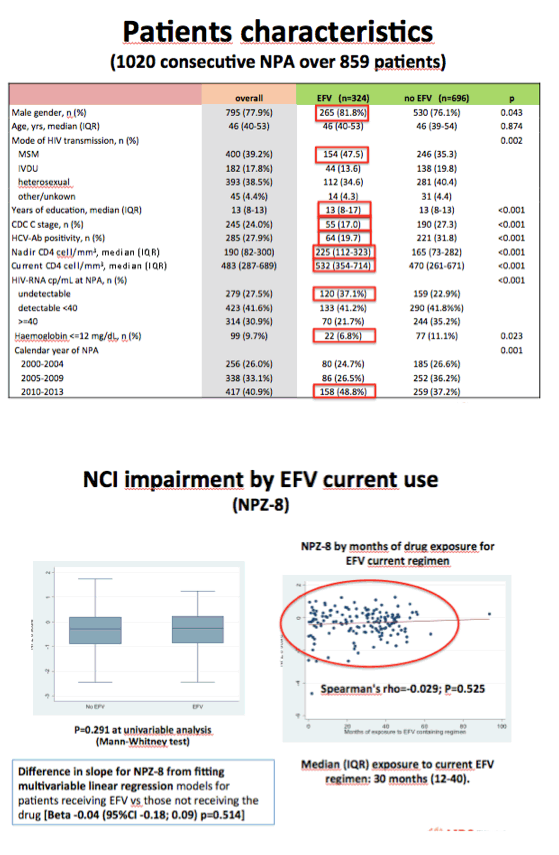
One third of neuropsychological assessments (32%) occurred when people were taking efavirenz, and the remaining assessments occurred when they were not taking efavirenz. Median time on the current antiretroviral regimen measured 21 months, and median time on efavirenz stood at 30 months. One quarter of the assessments (26%) were done in 2000-2004, one third (33%) in 2005-2009, and the rest (41%) in 2010-2013. Prevalence of NCI stood at 32.1% among people taking efavirenz and 39.9% among those not taking efavirenz.
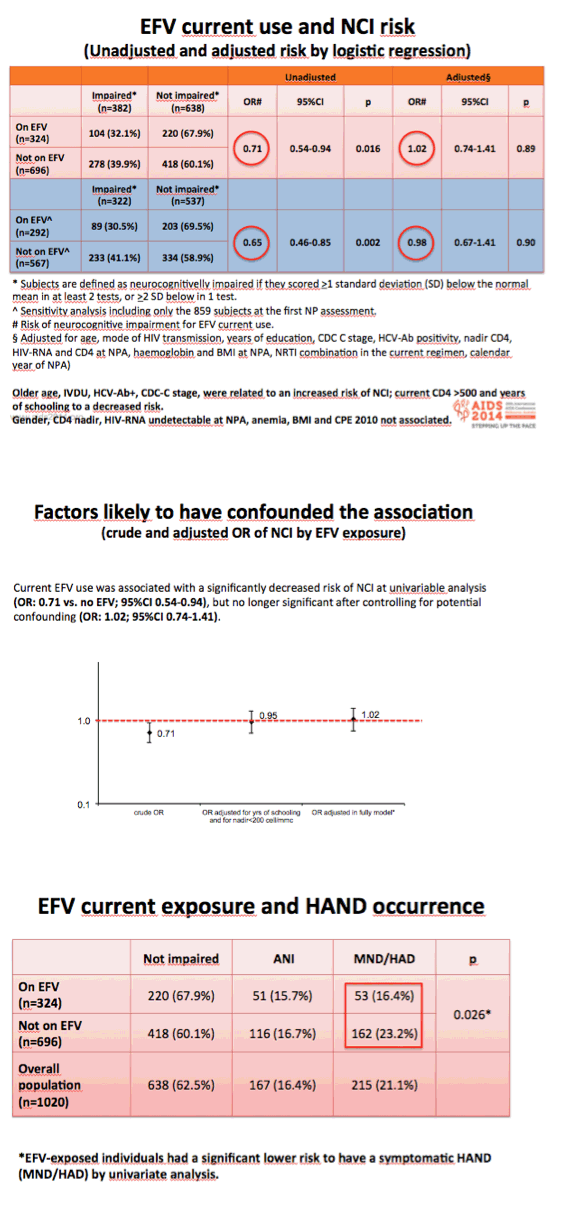
Univariate regression analysis linked taking efavirenz to almost 30% lower odds of neurocognitive impairment (odds ratio [OR] 0.71, 95% confidence interval [CI] 0.54 to 0.94, P = 0.016). But when the analysis included potential confounders, odds of neurocognitive impairment with current efavirenz were virtually even (OR 1.02, 95% CI 0.74 to 1.41, P = 0.89).
Multivariate logistic regression analysis identified six independent predictors of NCI, as indicated by the following adjusted odds ratios (aOR):
Higher chance of NCI:
Every additional 10 years of age: aOR: 1.34, 95% CI 1.15 to 1.55, P = 0.000
Injection drug use vs sex between men: aOR: 1.68, 95% CI 1.02 to 2.76, P = 0.040
CDC group C disease vs CDC A or B: aOR: 1.70, 95% CI 1.20 to 2.41, P = 0.003
HCV antibody positive: aOR 1.47, 95% CI 1.00 to 2.15, P = 0.049
Lower chance of NCI:
Each additional year of schooling: aOR 0.85, 95% CI 0.81 to 0.88, P = 0.000
CD4 count above 500 vs below 350 at neuropsychological testing: aOR 0.67, 95% CI 0.46 to 0.98, P = 0.041
Men tended to have higher odds of neuropsychological impairment than women (aOR 1.43, 95% CI 0.96 to 2.12, P = 0.078), as did people with hemoglobin at or below 12 mg/dL (aOR 1.60, 95% CI 0.98 to 2.60, P = 0.060). The 2010 central nervous system penetration effectiveness (CPE) score did not affect chances of NCI.
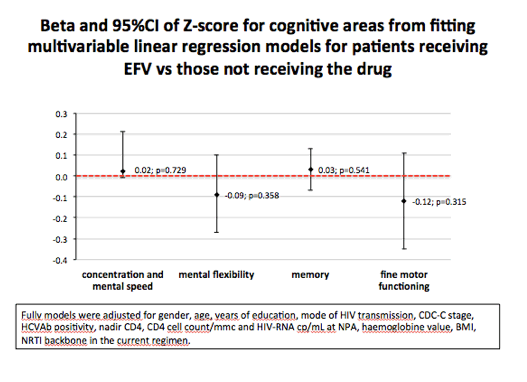
Study participants taking efavirenz had lower HAND prevalence than those not taking efavirenz (32.1% versus 39.9%, P = 0.026). But when the investigators analyzed these data only in neurocognitively impaired people, risk of HAND did not differ between the efavirenz group and the nonefavirenz group (P = 0.20). When they looked at specific neurocognitive domains, they found no difference between treatment groups.
"Even though confounding by indication may play a role, and reverse causality cannot be ruled out," the researchers concluded, "our results suggest that presence of NCI among persons treated with efavirenz-based cART is not more common than in people not treated with efavirenz."
References
1. Pinnetti C, Balestra P, Libertone R, et al. Use of efavirenz is not associated to an increased risk of neurocognitive impairment in HIV-infected patients. AIDS 2014. 20th International AIDS Conference. July 20-25, 2014. Melbourne. Abstract THAB0101.
2. Mollan KR, Smurzynski M, Eron JJ, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med.
2014;161:1-10. http://www.iasociety.org/Default.aspx?pageId=5&elementId=15898
3. Clifford DB, Evans S, Yang Y, et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143:714-721. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2958039/
----------------------


|
| |
|
 |
 |
|
|