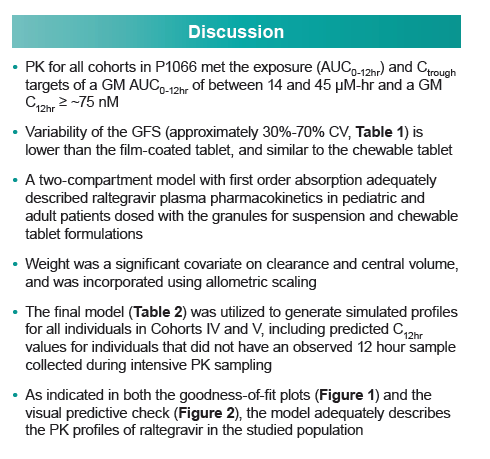
 |
 |
 |
| |
Population PK Modeling Supports Dosing of Two Pediatric Raltegravir Formulations
|
| |
| |
ICAAC 2014. September 5-9, 2014. Washington, DC
Mark Mascolini
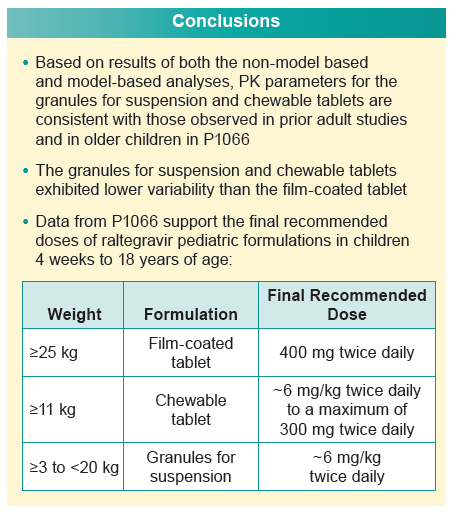
Data derived from population pharmacokinetic (PK) analysis of two raltegravir pediatric formulations support the following recommended doses for infants and children:
-- Weight 11 kg or higher: chewable tablet, approximately 6 mg/kg twice daily to maximum 300 mg twice daily
-- Weight 3 kg to under 20 kg: granules for suspension, approximately 6 mg/kg twice daily.
For children weighing 25 kg or more, the standard film-coated tablet should be given at 400 mg twice daily.
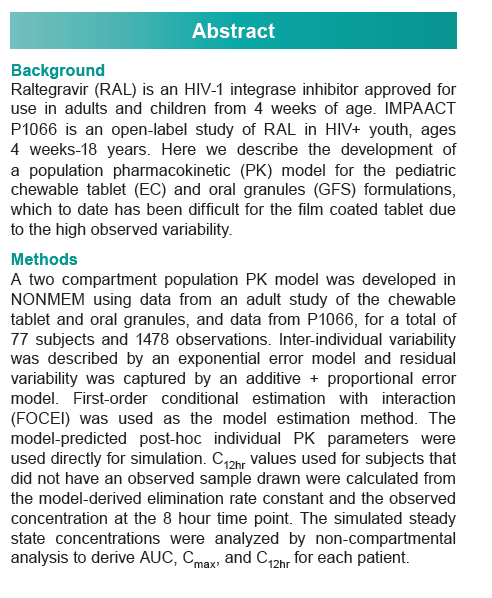
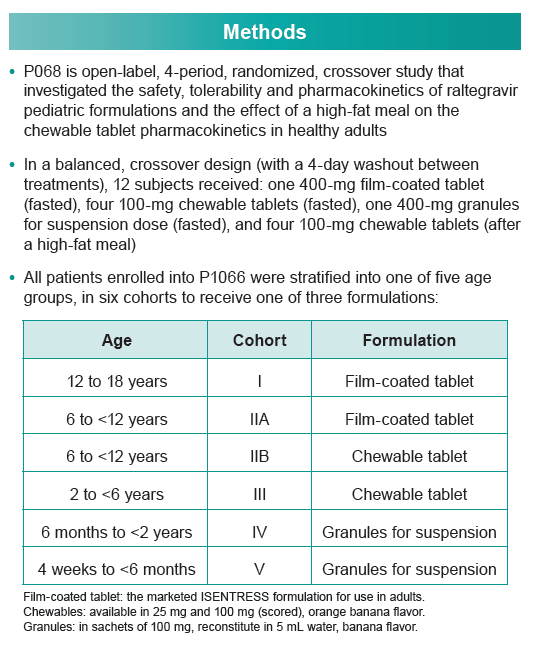
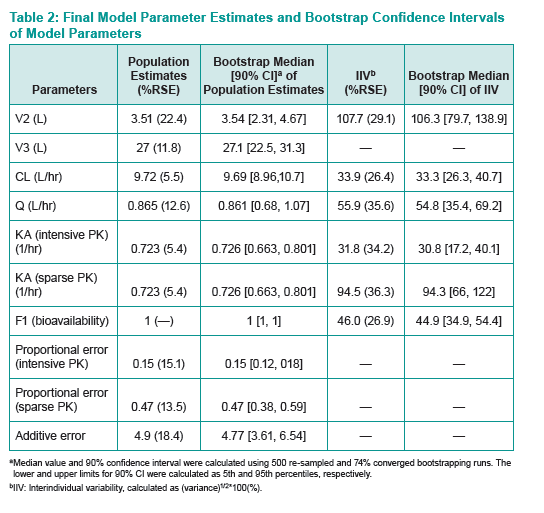
Raltegravir, the first integrase inhibitor, is licensed in the United States and Europe for children at least 4 weeks old and for adults. Developing a population PK model has proved difficult because of the high PK variability of the standard film-coated tablet. To address that problem, Merck investigators developed a two-compartment model from a healthy adult study of chewable tablets and oral granules (P068) and from IMPAACT P1066 in HIV-infected children.
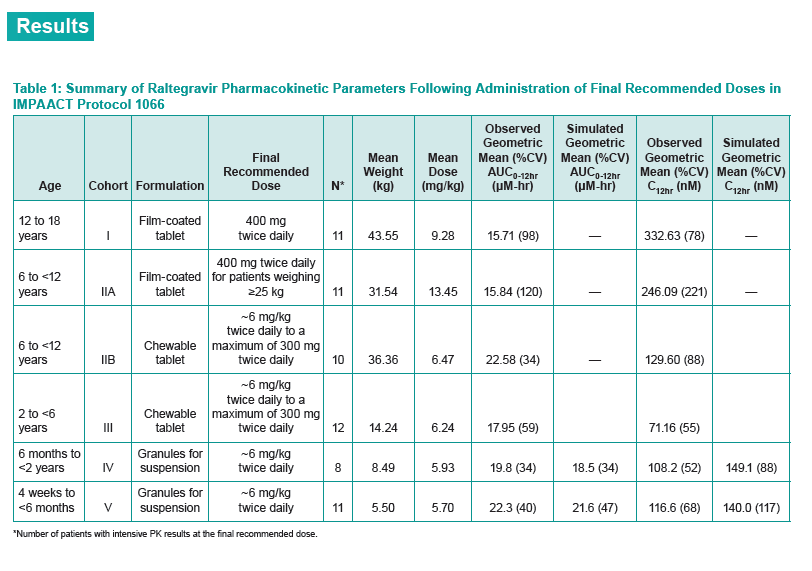
P068 investigated the safety, tolerability, and PKs of raltegravir pediatric formulations and the effect of a high-fat meal on chewable tablet PKs in healthy adults. IMPAACT P1066 was an open-label study of raltegravir in HIV-positive children from 4 weeks to 18 years old stratified into six groups: 12 to 18 (film-coated tablet), 6 to under 12 (film-coated tablet), 6 to under 12 (chewable tablet), 2 to under 6 (chewable tablet), 6 months to under 2 years (granules for suspension), 4 weeks to under 6 months (granules for suspension). The total analysis involved 1478 observations of 77 children and adults. The Merck team analyzed model-simulated steady-state concentrations to derive area under the concentration-time curve (AUC), maximum concentration (Cmax), and 12-hour concentration (C12h) for each person.
Each cohort in P1066 achieved targeted AUC (14 to 45 uM/h) and C12h (> 75 nM), results supporting selection of 6 mg/kg twice daily for the chewable tablet in children 2 to under 12 years old and for oral granules in infants and children 4 weeks to under 2 years old.
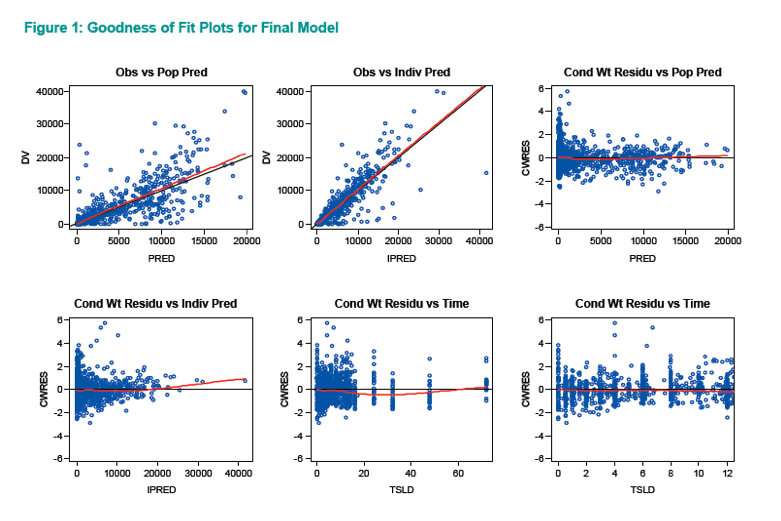
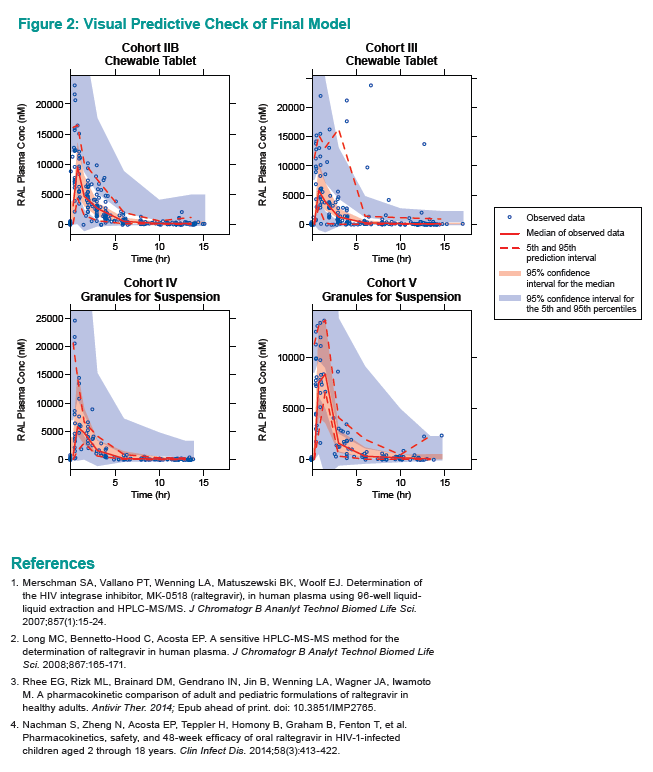
Goodness-of-fit plots and visual predictive checks indicated that the final model adequately describes raltegravir PKs in the target pediatric populations. The investigators reported the following observed and simulated geometric mean AUC0-12h and C12h for three pediatric groups:
4 weeks to under 6 months (granules for suspension)
Observed geometric mean AUC0-12h: 22.3 uM/h
Simulated geometric mean AUC0-12h: 21.6 uM/h
Observed geometric mean C12h: 116.6 nM
Simulated geometric mean C12h: 140.0 nM
6 months to under 2 years (granules for suspension)
Observed geometric mean AUC0-12h: 19.8 uM/h
Simulated geometric mean AUC0-12h: 18.5 uM/h
Observed geometric mean C12h: 108.2 nM
Simulated geometric mean C12h: 149.1 nM
2 years to under 6 year (chewable tablet)
Observed geometric mean AUC0-12h: 17.95 uM/h
Observed geometric mean C12h: 71.16 nM
PK variability of oral granules (about 30% to 70% coefficient of variation) was similar to that of chewable tablets and lower than that of film-coated tablets.
The researchers concluded that results of both model-based and non-model-based analyses indicate that PKs of granules for suspension and chewable tablets are consistent with those seen in previous adult studies and in older children in study P1066.
Reference
1. Rizk M, Du L, Wenning L, et al. Population pharmacokinetic analysis of raltegravir pediatric formulations in HIV-infected children 4 weeks to 18 years of age. ICAAC 2014. September 5-9, 2014. Washington, DC. Abstract H-1013a.
------------------------------
Population Pharmacokinetic Analysis of Raltegravir Pediatric Formulations in HIV-Infected Children 4 Weeks to 18 Years of Age
Reported by Jules Levin, NATAP
ML Rizk1, L Du1, L Wenning1, C Bennetto-Hood2, and EP Acosta2
1Merck & Co., Inc., Whitehouse Station, NJ, USA; 2University of Alabama-Birmingham, Birmingham, AL, USA


|
| |
|
 |
 |
|
|