 |
 |
 |
| |
Subgroup Analysis of Response to Stribild vs Atripla or Atazanavir: 144 Weeks
|
| |
| |
ICAAC 2014. September 5-9, 2014. Washington, DC
Mark Mascolini
Overall response to Stribild generally matched responses to Atripla or an atazanavir/ritonavir regimen in 144-week subgroup analyses of two randomized trials [1]. Certain adverse events proved less frequent with Stribild than with the other two regimens.
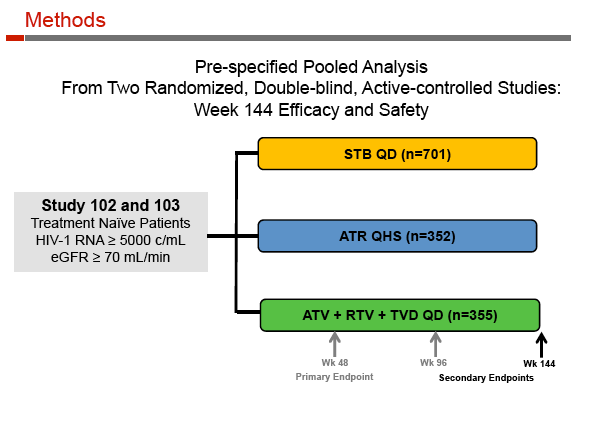
Stribild, a once-daily single-tablet regimen containing the integrase inhibitor elvitegravir boosted by cobicistat plus tenofovir/emtricitabine (TDF/FTC), is licensed for antiretroviral-naive adults. In separate randomized trials, Stribild proved noninferior to Atripla (coformulated efavirenz plus TDF/FTC) [2] and atazanavir/ritonavir plus TDF/FTC [3]. The 144-week analysis involved 1408 participants in those two trials, 701 randomized to Stribild, 352 to Atripla, and 355 to atazanavir.
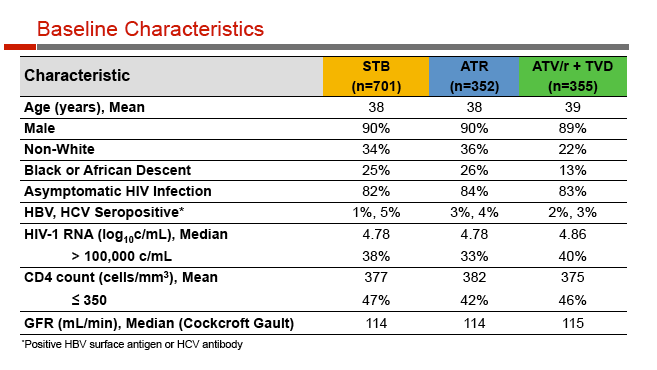
Age averaged 38 in the three groups, 90% were men, and about one third were nonwhite. Pretreatment CD4 counts averaged 377 with Stribild, 382 with Atripla, and 375 with atazanavir. Respective pretreatment viral loads were 4.78 log, 4.78 log, and 4.86 log (about 60,000 to 72,000 copies).
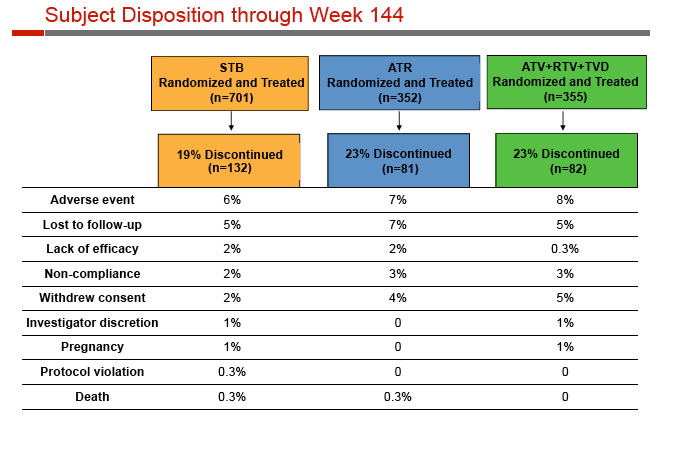
While 19% discontinued Stribild, 23% stopped Atripla, and 23% stopped the atazanavir regimen. Respective rates of stopping for lack of efficacy were 2%, 2%, and 0.3%.
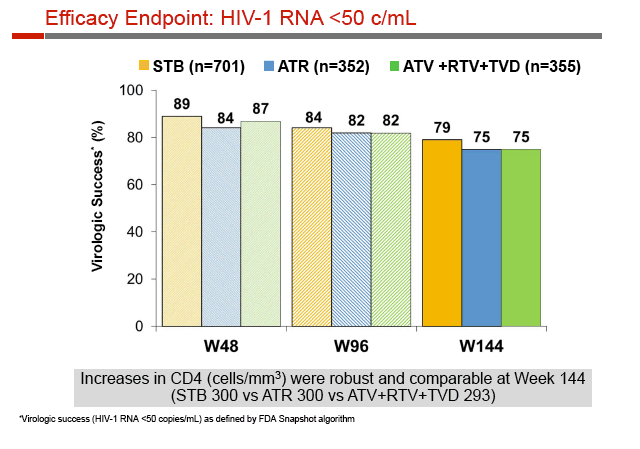
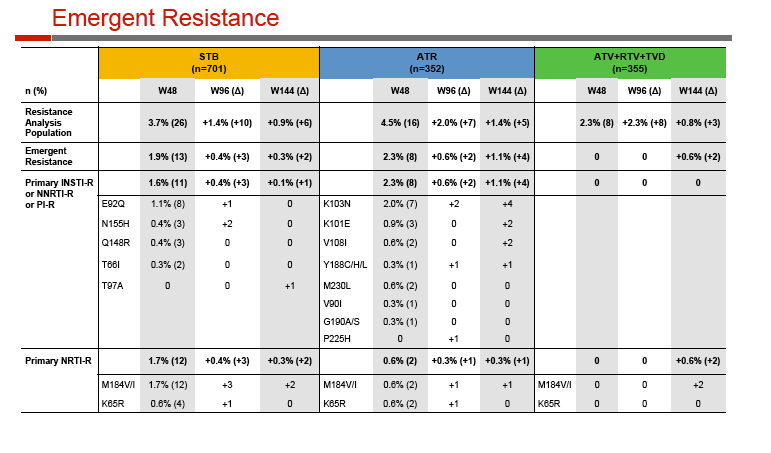
At week 144, 79% randomized to Stribild, 75% to Atripla, and 75% to atazanavir had a viral load below 50 copies. Among participants with virologic failure, emergence of resistant virus was least frequent with atazanavir (0.6%), intermediate with Stribild (2.6%), and highest with Atripla (4.0%).
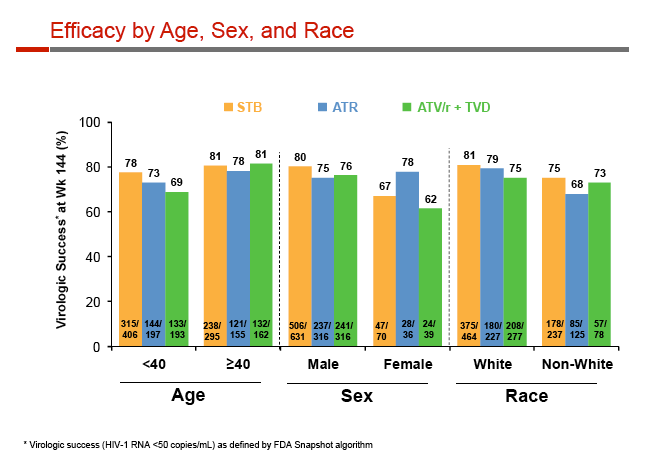
Virologic response rates were similar among participants 40 or older (Stribild 81%, Atripla 78%, atazanavir 81%) or younger than 40 (78%, 73%, 69%). The response rate was modestly greater with Stribild than the other two regimens among men (80%, 75%, 76%) and moderately lower with Stribild than Atripla among women (67%, 78%, 62%), but the analysis included only 36 women taking Atripla.
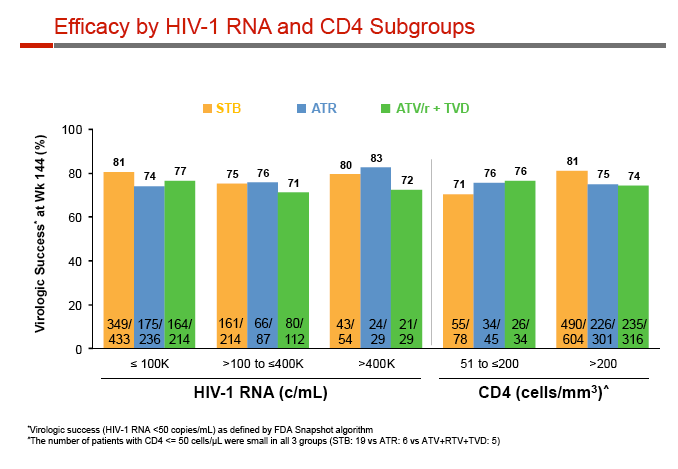
Sub-50-copy rates at 144 weeks were similar with Stribild, Atripla, and atazanavir among whites (81%, 79%, 75%) and nonwhites (75%, 68%, 73%), and among people with a pretreatment viral load above 100,000 copies (75%, 76%, 71%) or a CD4 count below 200 (71%, 76%, 76%). Because of smaller numbers in the analysis, 144-week response rates with Stribild, Atripla, and atazanavir were slightly more variable among people with a baseline viral load above 400,000 copies (80%, 83%, 72%).
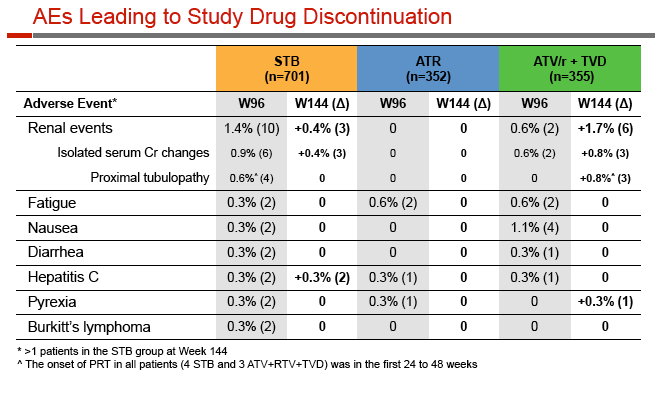
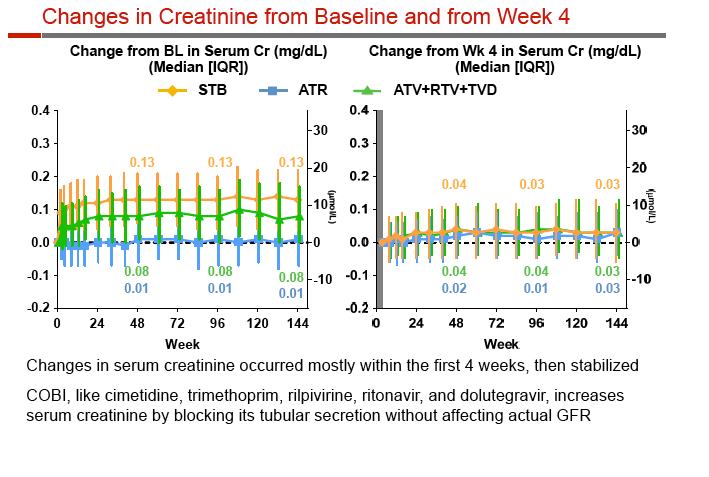
Rates of adverse events leading to discontinuation were similar with Stribild, Atripla, and atazanavir (6.0%, 7.4%, 8.5%), and renal-related discontinuation was rare (1.9%, 0, 2.3%). Among people taking Stribild, 4 (0.6%) had proximal renal tubulopathy in the first 24 weeks and none after that, while 3 cases (0.8%) developed through 48 weeks among people taking atazanavir. Median serum creatinine changes through 144 weeks were +0.13 mg/dL with Stribild, +0.01 mg/dL with Atripla, and +0.08 mg/dL with atazanavir; those rates had not changed substantially since week 4.
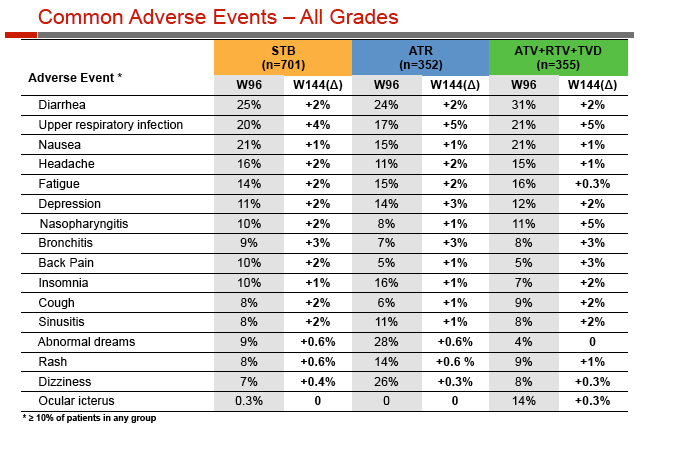
Through 144 weeks, significantly fewer people taking Stribild than Atripla had neuropsychiatric side effects (47.2% versus 67.9%, P < 0.001) or rash (24.3% versus 31.8%, P = 0.01). A lower proportion of people randomized to Stribild than to atazanavir had diarrhea (26.8% versus 33.2%, P = 0.03).
References
1. Elion R, Squires K, Bloch M, et al. Subgroup analyses of 144-week efficacy and safety of elvitegravir/cobicistat/emtricitabine/tenofovir DF (EVG/COBI/FTC/TDF). ICAAC 2014. September 5-9, 2014. Washington, DC. Abstract H-644.
2. Zolopa A, Sax PE, DeJesus E, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;63:96-100.
3. Rockstroh JK, DeJesus E, Henry K, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62:483-486.
----------------------
Pooled 144-week Efficacy and Safety of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir DF (STB) with Subgroup Analysis
Reported by Jules Levin
ICAAC Sep 5-9, 2014
54th Interscience Conference on Antimicrobical Agents and Chemotherapy
Washington, DC
Richard Elion1, Kathleen Squires2, Mark Bloch3, Michael Sension4, Michael Wohlfeiler5, Joanne Curley6, Lijie Zhong6, Marshall Fordyce6, Martin S Rhee6, Javier Szwarcberg6
Whitman Walker Health, Washington, USA1, Thomas Jefferson University, Philadelphia, USA 2, Holdsworth House Medical Practice, Darlinghurst, Australia3, Comprehensive Care Center, Ft. Lauderdale, USA4, Wohlfeiler, Piperato and Associates, LLC, Miami Beach, USA5, Gilead, Foster City, USA6
|
| |
|
 |
 |
|
|