 |
 |
 |
| |
Virologic Response During ART Interruption After CCR5-Cell Modification With SB-728-T (zinc fingers)
|
| |
| |
full slide & poster data presentations & Sangamo press release below
ICAAC 2014. September 5-9, 2014. Washington, DC
Mark Mascolini
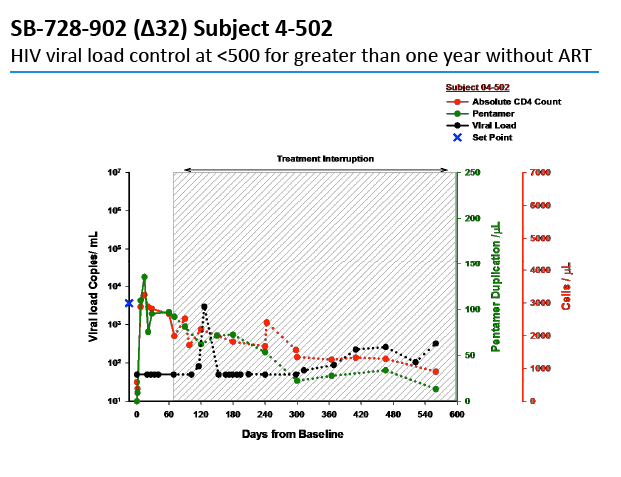
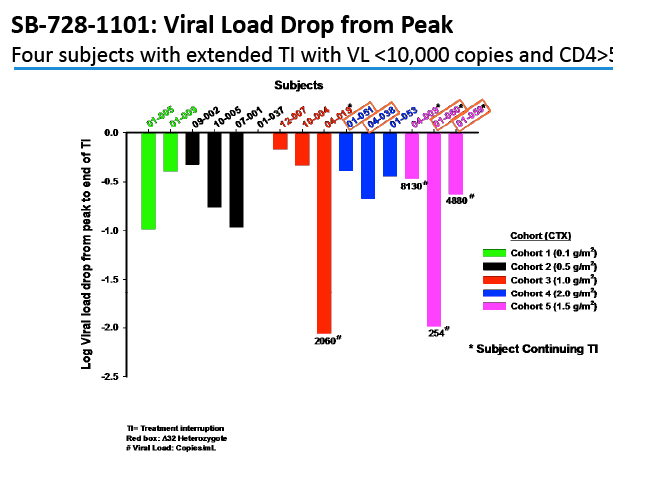
After interruption of antiretroviral therapy (ART), 2 people with infused SB-728-T-modified CD4 cells had a 10-fold drop in viral load, and 2 had a 100-fold drop [1]. Four people in this trial continue their antiretroviral interruption with a viral load below 10,000 copies.
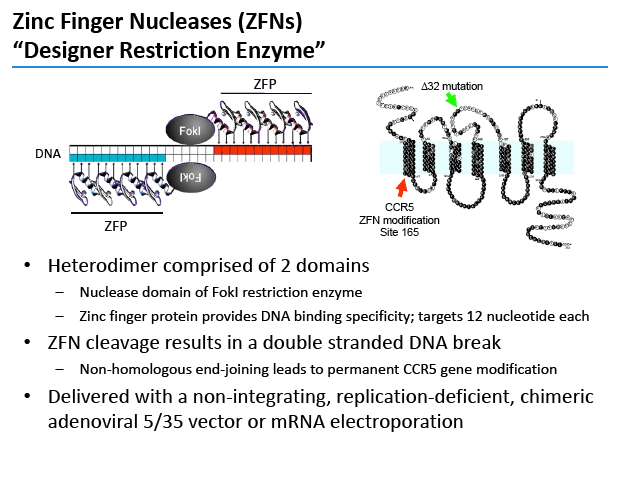
SB-728-T is a zinc-finger DNA-binding protein transcription factor targeting the gene that makes CCR5 coreceptors on CD4 cells [2,3]. Cells lacking CCR5 receptors ("CCR5 knockouts") resist infection with CCR5-using HIV. Researchers at Sangamo BioSciences, developer of SB-728-T, hope that CD4-cell modification with this novel agent will foster development of a new CD4-cell population resistant to HIV and thus perhaps allow control of HIV without antiretroviral therapy.
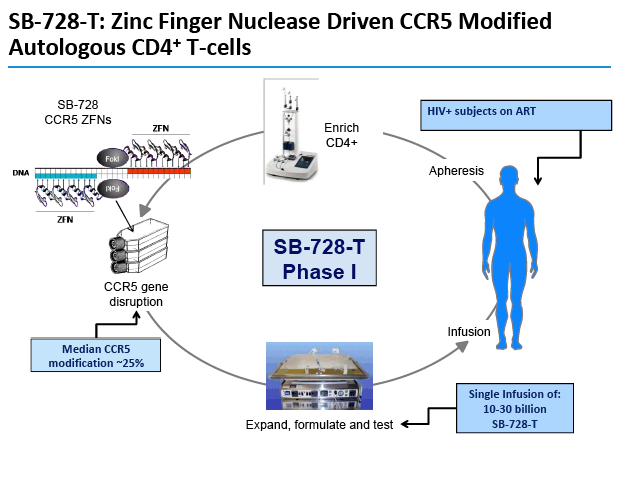
Treatment begins with collection of CD4 cells from an HIV-positive person [3]. Exposure of these cells to SB-728-T aims to yield a CD4 population with nonfunctional CCR5 coreceptors. The modified CD4-cell population is stimulated to produce more CCR5-disabled CD4 cells, which are then infused into the HIV-infected donor.
People homozygous for the CCR5 delta-32 deletion resist infection with CCR5-using HIV because their CD4 cells lack the CCR5 coreceptor. People heterozygous for the CCR5 delta-32 deletion have slower HIV progression than people without the deletion. In a previous study 1 of 4 people who could be evaluated achieved an undetectable viral load during a 12-week antiretroviral interruption after SB-728-T CD4-cell modification [4]. This person was a CCR5 delta-32 heterozygote.
In that study median CD4 count rose from 448 to 1517 a week after infusion of SB-728-T-modified cells [4]. Median number of modified cells at 1 week was 250, representing 9% of circulating peripheral blood mononuclear cells and 14% of circulating CD4 cells. One serious adverse event--an infusion reaction--occurred with infusion of modified cells.
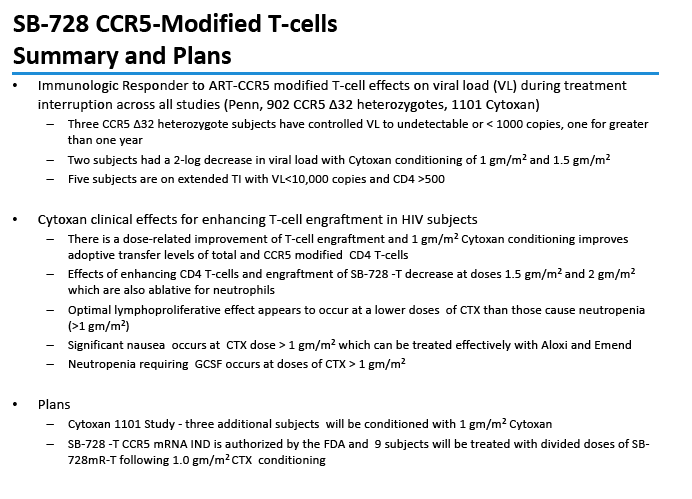
The study presented at ICAAC involved 18 people in five SB-728-T dosage groups who had cyclophosphamide (Cytoxan) conditioning before the procedure [1]. The investigators noted that Cytoxan has beneficial immune-modulating effects in T-cell adoptive transfer immunotherapy, such as type 1 interferon production, dendritic cell activation with antigen-driven T-cell proliferation, and improved homing to lymphoid organs.
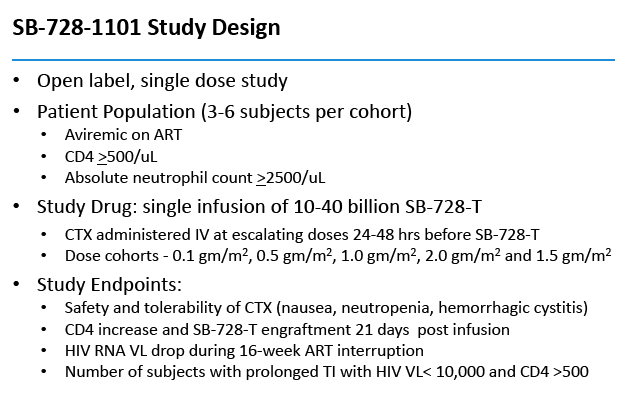
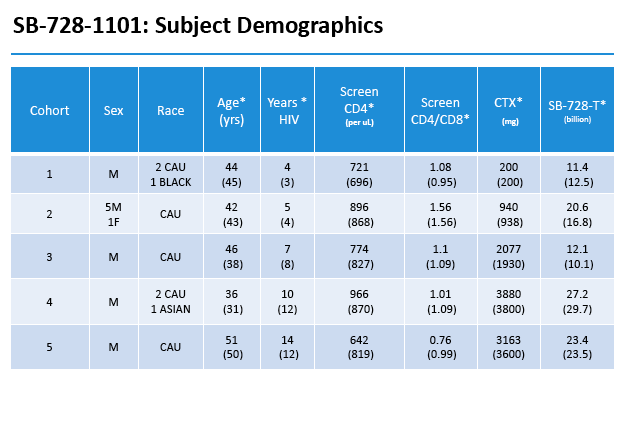
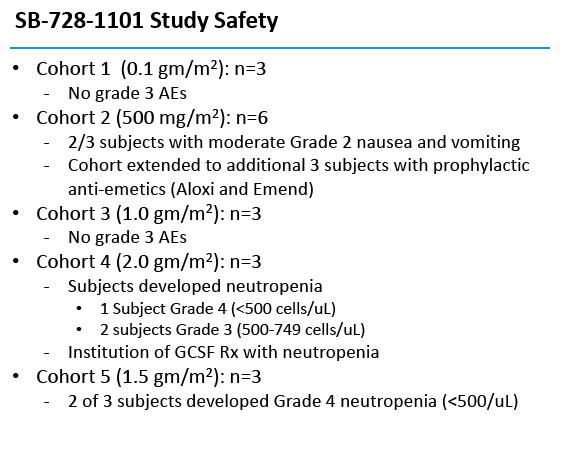
This open-label single-dose study enrolled people who had attained an undetectable viral load with antiretroviral therapy, had a CD4 count above 500, and had an absolute neutrophil count above 2500. The five doses were 0.1, 0.5, 1.0, 1.5, and 2.0, g/m(2). Six people received the 0.5 g/m(2) dose, while 3 each received the other doses. Participants received intravenous Cytoxan at escalating doses 24 to 48 hours before SB-728-T.
All but 1 study participant were men and all were Caucasian except for 1 black and 1 Asian. Median age ranged from 36 to 51 across the five dosage cohorts, while median duration of HIV infection ranged from 4 to 12 years.
Two of the three initial participants in the 0.5-g/m(2) cohort had grade 2 nausea and vomiting, which antiemetics controlled. The researchers then added 3 people to this cohort, all of them taking prophylactic antiemetics. One person in the 2.0 g/m(2) group had grade 4 neutropenia (under 500 neutrophils) and 2 had grade 3 neutropenia (500 to 749 neutrophils). Two of 3 people in the 1.5-g/m(2) cohort had grade 4 neutropenia. There were no grade 3 or 4 adverse events in the other two dosage cohorts.
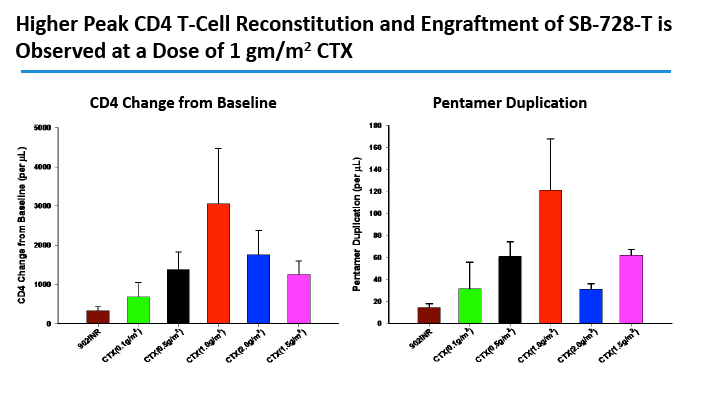
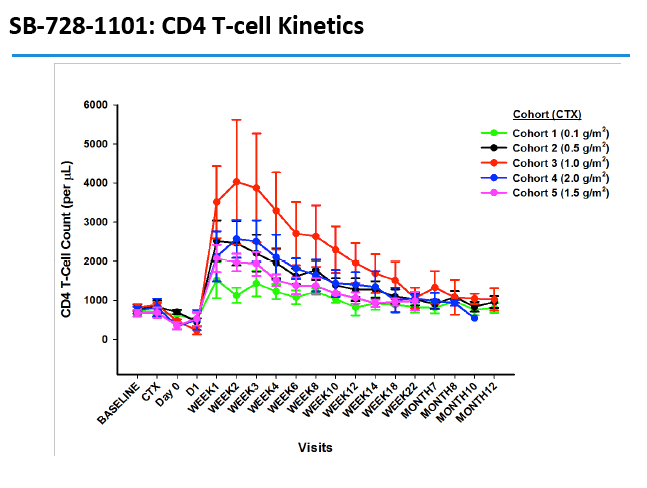
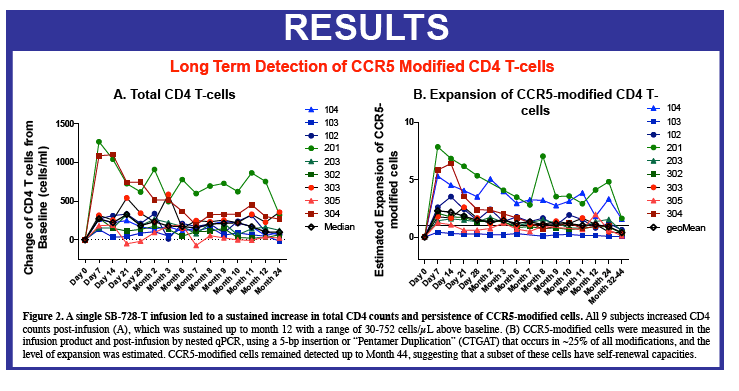
CD4 counts rose in all 5 dosage groups and by more than 1000 cells in four of the five groups. The gain was not dose proportional, as the biggest jump (to 4000 cells) occurred in the 1.0-g/m(2) group.
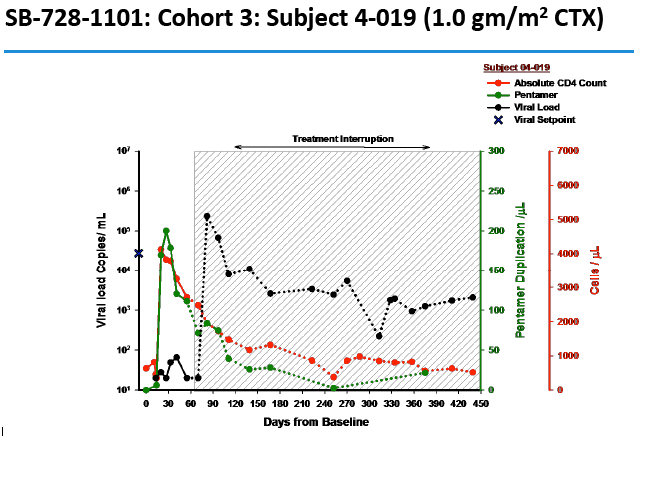
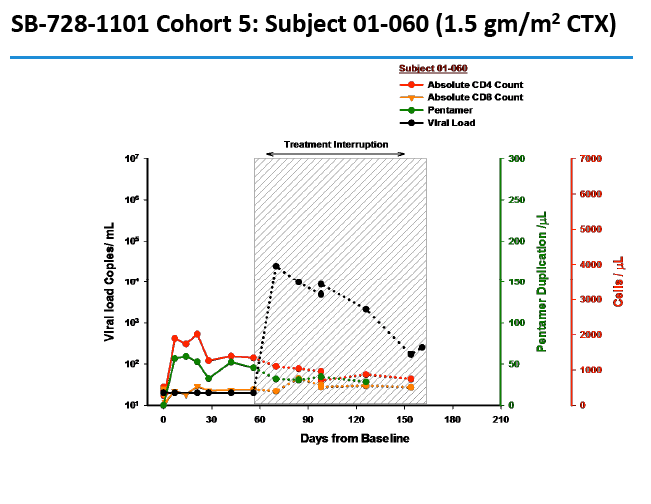
Two people achieved a 1-log (10-fold) drop in viral load, while 2 attained a 2-log (100-fold) drop. The two people with a 100-fold decline in viral load received the 1.0- and 1.5-g/m(2) doses. Four people, including the 2 with the 100-fold decline, were continuing their antiretroviral interruption at the time of this report with a viral load below 10,000 and a CD4 count above 500.
References
1. Ando D, Lalezari J, Blick G, et al. HIV protected autologous zinc finger nuclease CCR5 modified CD4 cells (SB-728-T) reduce viral load in HIV subjects during treatment interruption: correlates of effect, and effect of Cytoxan conditioning. ICAAC 2014. September 5-9, 2014. Washington, DC. Abstract H-643.
2. AIDSMEDS. SB-728-T. http://www.aidsmeds.com/archive/SB-728-T_2574.shtml ...... http://www.natap.org/2009/newsUpdates/122909_01.htm
3. AIDSinfo. SB-728-T. AIDSinfo Drug Database. http://aidsinfo.nih.gov/drugs/532/sb-728-t/0/patient
4. Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901-910. http://www.natap.org/2014/HIV/041014_05.htm
September 8, 2014
TWO SLIDES/POSTER PRESENTATIONS BELOW
Sangamo BioSciences Announces Presentation At ICAAC of New Clinical Data Demonstrating Sustained Functional Control of Viremia in Multiple HIV-Infected Subjects Treated with SB-728-T
Control of Viral Load During Treatment Interruption of Antiretroviral Therapy Sustained and Ongoing for More than One Year in One Subject and Several Months in Another
Additional Presentation Demonstrates Potential Mechanisms for Unprecedented HIV Reservoir Depletion by SB-728-T
RICHMOND, Calif., Sept. 8, 2014 /PRNewswire/ -- Sangamo BioSciences, Inc. (Nasdaq: SGMO) announced the presentation of new data demonstrating that a single infusion of Sangamo's novel ZFP Therapeutic®, SB-728-T, resulted in sustained reduction and control of viral load (VL) in the absence of antiretroviral drugs (ART) in several subjects. In addition, a decrease in the size of the HIV reservoir, as demonstrated by measurement of HIV total DNA in peripheral blood mononuclear cells (PBMCs), was observed over a three year period in nine of nine subjects treated who remained on ART throughout the study. These decreases, in some cases a two to three-log reduction, are in remarkable contrast to the stable levels typically seen with ART treatment alone.

The data were generated in clinical trials designed to evaluate safety and efficacy of SB-728-T for the treatment of HIV/AIDS, and were presented at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) on Sunday, September 7, 2014.
In an oral presentation, Dale Ando, M.D., Sangamo's vice president of therapeutic development and chief medical officer, described data from two studies (SB-728-902 Cohort 5 and SB-728-1101) designed to maximize the engraftment of ZFN modified CD4+ T-cells (SB-728-T) in which both copies of the CCR5 gene had been disrupted, making these cells fully resistant to HIV infection. In total, across all trials that Sangamo has conducted, three CCR5 delta 32 heterozygote subjects have controlled VL to undetectable or < 1000 copies during a treatment interruption (TI) from ART, one for more than 59 weeks (to last measurement taken). In the 1101 study, two subjects have experienced a two-log decrease in viral load from peak (with Cytoxan conditioning of 1 gm/m2 and 1.5 gm/m2) which has been sustained in one subject for more than 39 weeks. Five subjects currently remain on extended TI (longer than the 16 week period defined in the protocol) with VLs < 10,000 copies and CD4 counts of > 500.
"We have now demonstrated profound suppression of viral load in the blood and sustained functional control of the virus in multiple subjects treated with just a single infusion of SB-728-T," stated Dr. Ando. "In contrast, while there have been previous reports of functional control in two subjects who received bone marrow transplants from uninfected individuals, none of these individuals was able to maintain control of their viral load for longer than a few weeks in the absence of ART. Subjects on our trials have significantly surpassed that milestone. In addition, our data also suggest potential mechanisms for this immunologic control of the virus and the all important reservoir, which must be depleted in order to achieve lasting functional control of HIV in infected individuals."
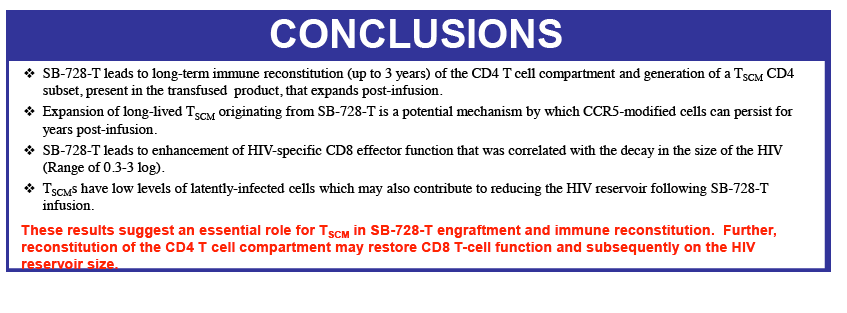
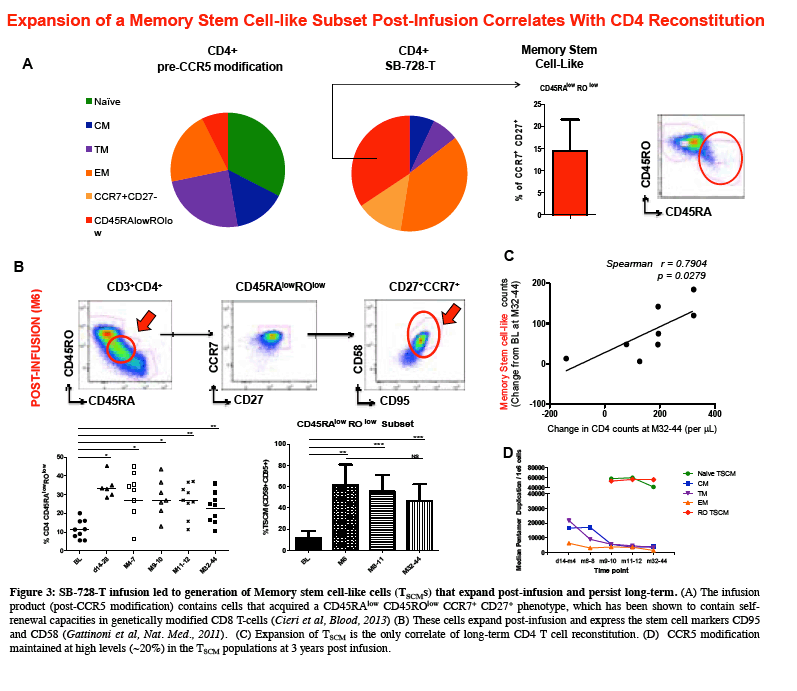
"Sangamo's data to date suggest that SB-728-T treatment can enable an individual's immune system to attack HIV infection from two directions: by controlling actively replicating virus in the blood and by reducing the latent HIV reservoir which turns over more slowly," said Geoff Nichol, M.B., Ch.B., Sangamo's executive vice president of research and development. "Our aim is to provide a protected reservoir of immune memory cells to replenish the cells killed by HIV and to generate an effective immune response against the virus and opportunistic infections. SB-728-T treatment has resulted in an unprecedented and durable increase in CD4+ cells, which is likely to be primarily due to the expansion of protected CD4+ stem cell central memory' cells (TSCM) which are self-renewing stem-like cells. We postulate that protection of TSCM from infection provides two possible routes to limit, and ultimately control HIV in an infected individual. The protected CD4+ cells that TSCM generates can provide sustained anti-HIV immune helper function against infected cells, allowing functional control of virus in the blood and reservoir and, as these TSCM cannot be infected, their presence ultimately limits the ability of the reservoir to be replenished and maintained which may, over time, result in reservoir erosion."
Some HIV-infected individuals, so-called 'elite controllers', can accomplish this without drug intervention. These individuals typically have low CCR5 expression and good anti-viral CD8 responses, a characteristic shared by those SB-728-T treated subjects in which the greatest effects on the virus have been seen to date.
With the recent acceptance of an IND for mRNA delivery of ZFNs Sangamo is conducting an ongoing Phase 2 clinical trial, SB-728-mR-1401 (1401), designed to provide further evidence of functional control of HIV in additional subjects. The protocol incorporates a number of methods to increase the engraftment of CD4 T-cells that have undergone biallelic CCR5 gene modification, including certain criteria for subject selection, optimal Cytoxan preconditioning and a number of process improvements such as mRNA delivery of the ZFNs, which will allow the administration of multiple doses of the modified cells. In addition to a further three subjects treated at the optimal dose of Cytoxan using adenoviral delivery of ZFNs, the Company expects to enroll all nine subjects into the 1401 study by the end of 2014.
"The data presented at ICAAC, continue to support our conviction that an immunologic approach to a functional cure of HIV is likely to be the most successful," added Dr. Nichol. "The SB-728-mR-1401 study combines our best understanding, from all of our clinical research, of the potential mechanism of this novel therapeutic and we believe that it will yield data that provide a clear path to pivotal studies."
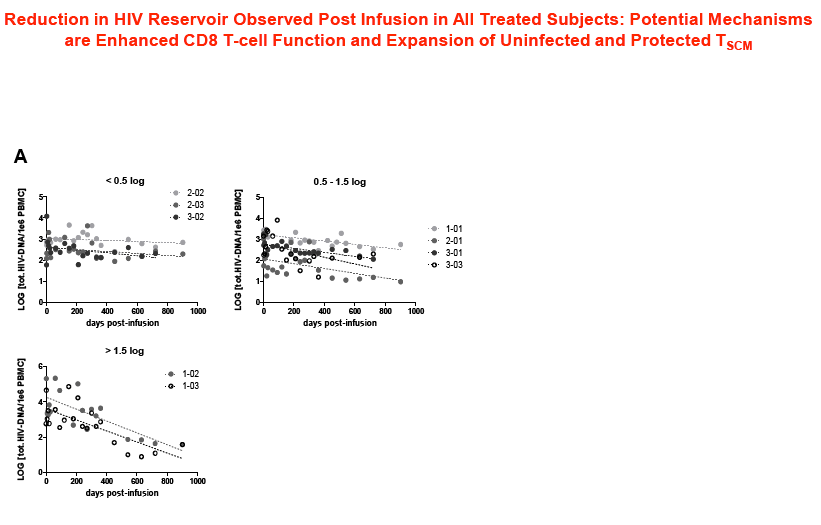
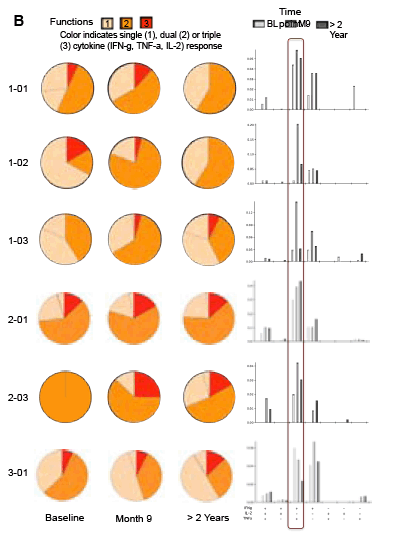
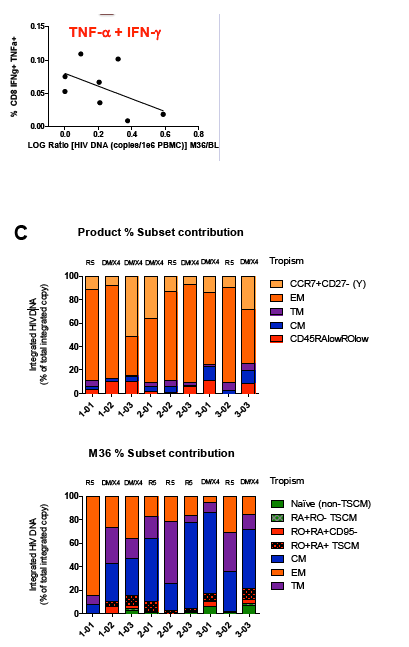
Sangamo's collaborators from the laboratory of Rafick-Pierre Sékaly, Ph.D., Co-Director & Chief Scientific Officer of the Vaccine & Gene Therapy Institute of Florida (VGTI Florida) also presented analyses of immunological data from SB-728-T-treated subjects (SB-728-902 Cohorts 1-3). They demonstrated a large increase in CCR5-modified cells in the long-lived TSCM compartment which may explain why CCR5-modified cells from a single infusion can be detected in all subjects over a prolonged period (more than 42 months). A median 0.9 log decrease in the size of the HIV reservoir at 36 months was observed in nine of nine subjects treated, as demonstrated by measurement of HIV total DNA in PBMCs. The decrease in reservoir showed a statistically significant correlation with an improvement in CD4 count. TSCM is thought to be the principal cell-type that comprises the HIV reservoir, which is a source of HIV that is maintained in infected individuals in the form of the HIV genome integrated into the CD4 T-cell's DNA. These HIV-carrying cells can be found throughout the body, in the blood, and in larger numbers in the lymph system. The reservoir cannot be diminished by ART which only inhibits the growth of actively replicating virus in the blood. When an HIV-infected individual with well-controlled virus stops taking ART, the reservoir serves as a source of HIV and the VL in the blood quickly rises before reaching a plateau at or around the viral set point. The data demonstrate that SB-728-T treatment is associated with reduction in both the VL and the levels of the reservoir.
About SB-728-T
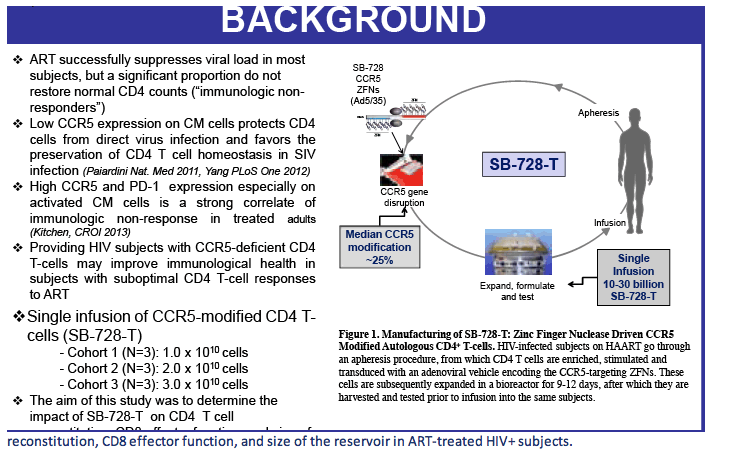
Sangamo's drug, SB-728-T, is generated by ZFN-mediated modification of the gene encoding CCR5 in a patient's own T-cells. ZFN modification disrupts the expression of this key co-receptor for HIV entry and renders cells resistant to HIV infection. The approach is based on the observation that a naturally occurring mutation in the CCR5 gene, CCR5 delta-32, provides protection from HIV infection. Individuals in whom both copies of the CCR5 gene carry the delta-32 mutation are generally not susceptible to the most common strain of HIV.
Summary of Clinical Trial Design
About SB-728-902 Cohorts 1-3
The study was an open-label Phase 1 clinical trial to evaluate the safety and tolerability of single infusions of an escalating dose of an autologous (a patient's own) CD4+ T-cell product genetically modified at the CCR5 gene by CCR5-specific ZFNs (SB-728-T). The trial enrolled nine HIV-infected subjects (three cohorts of three subjects each) who have sub-optimal T-cell levels and no detectable viral load on long-term ART, so-called immunologic non-responders (INRs). Subjects remained on their existing antiviral therapy while receiving treatment with SB-728-T.
About SB-728-902 Cohort 5
HIV-infected subjects heterozygous for the CCR5 delta-32 mutation (i.e. with one CCR5 gene that is naturally modified) who are currently on ART were enrolled and received a single intravenous infusion of SB-728-T (5 to 30 billion modified cells). Two months after SB-728-T treatment, subjects underwent a 16 week TI during which time their ART was discontinued. ART was reinstituted in subjects whose CD4 T-cell counts dropped, or whose HIV-RNA increased to pre-defined levels. At the end of the TI, subjects with a sustained detectable HIV viral load were reinstituted on ART. Subjects with a significantly reduced viral load were given the option of remaining off ART until HIV RNA levels rose significantly and/or their CD4 T-cell count dropped to pre-defined levels. A total of ten subjects were treated in this cohort.
About SB-728-1101 Cohorts 1-5
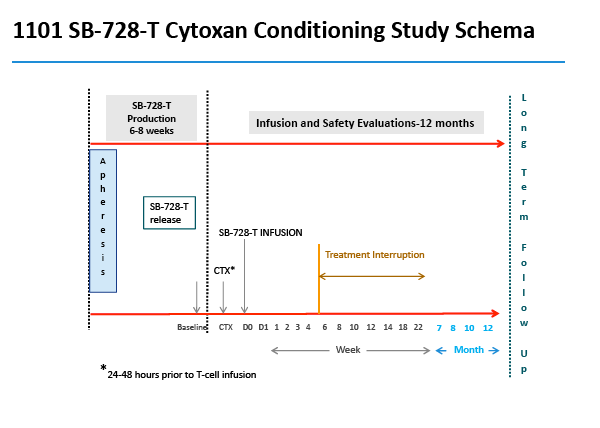
SB-728-1101 is an open-label, dose escalation, multi-center study designed primarily to evaluate the safety and tolerability of escalating doses of cyclophosphamide (Cytoxan®) administered prior to SB-728-T infusion. Cytoxan is a drug that is used to transiently reduce the numbers of T-cells in the body, which then rapidly repopulate once the drug is discontinued, and it is into this "growth" environment that SB-728-T is infused. Such lymphodepletive treatment has been used to enhance engraftment of adoptively transferred T-cells in the treatment of cancer, and as therapy for numerous autoimmune diseases. The drug has been previously used in HIV-infected individuals and studies demonstrate that while the drug was transiently lymphodepleting, it did not significantly reduce total CD4 T-cell counts over the long term and was adequately tolerated.
In addition to safety, the study is designed to evaluate the effect of escalating doses of Cytoxan on SB-728-T engraftment, the effect of SB-728-T treatment on viral load following ART interruption, the change in CD4+ T-cell counts in peripheral blood and the long-term persistence of SB-728-T.
By protocol, HIV-infected subjects on ART were enrolled into five dose-escalating cohorts (three subjects/cohort), and received intravenous Cytoxan (200 mg, 500 mg/m2, 1.0g/m2, 1.5g/m2 and 2.0g/m2). In cohort two, an additional three subjects were evaluated on an improved anti-emetic protocol due to an adverse event of Grade 2 nausea observed in two subjects at that dose level. Within each cohort, treatment was staggered so that each subsequent subject was infused with Cytoxan two weeks after the preceding subject. One to three days after receiving Cytoxan, subjects were infused with SB-728-T (8.2 to 36.2 billion cells). Six weeks after SB-728-T infusion, subjects with CD4 cell counts ≥500 cells/mm3 underwent a 16 week TI during which time their anti-retroviral therapy was discontinued. ART was reinstituted in subjects whose CD4 T-cell counts dropped and/or whose HIV-RNA increased to certain pre-defined levels. At the end of the TI, subjects with a sustained detectable viral load or reduced CD4 T-cell count were reinstituted on ART. However, subjects who had experienced a drop in viral load were given the option of remaining off ART until HIV-RNA levels rose and/or their CD4 T-cell count dropped below pre-defined levels.
About SB-728-mR-1401
SB-728-mR-1401 is an open-label, multi-center study designed primarily to evaluate safety and tolerability and the effect of repeat doses of SB-728-T following optimal cyclophosphamide pre-conditioning, on engraftment, viral load and total CD4 counts in peripheral blood. The study will use a new improved process, using electroporation of mRNA encoding the ZFNs, rather than an adenoviral vector to deliver the ZFNs to the isolated T-cells. This process enables repeat dosing of the product. Up to nine subjects will be enrolled into two cohorts. Each subject will receive a total of up to 40 billion ZFN modified T-cells. The first cohort will receive this dose divided into infusions of two equal doses of SB-728mR-T 14 days apart after a cyclophosphamide (1 g/m2) preconditioning treatment two days prior to the first SB-728mR-T infusion, and the second cohort will receive three doses of cells. Dividing the total cell dose and administering sequentially in this manner is thought to maximize overall cell engraftment. Four weeks after the last SB-728-mR infusion, subjects with CD4 cell counts ≥500 cells/mm3 will undergo a 16 week TI during which time their anti-retroviral therapy will be discontinued. ART will be reinstituted in subjects whose CD4 T-cell counts drop and/or whose HIV-RNA increases to certain pre-defined levels. At the end of the TI, subjects with a sustained detectable viral load or reduced CD4 T-cell count will be reinstituted on ART. However, subjects who experience a drop in viral load will be given the option of remaining off ART until HIV-RNA levels rise and/or their CD4 T-cell count drops below pre-defined levels.
--------------
HIV Protected AutologousZinc Finger Nuclease CCR5 Modified CD4 cells (SB-728-T) Reduce Viral Load (VL) in HIV Subjects During Treatment Interruption (TI): Correlates of Effect, and Effect of CytoxanConditioning
Reported By Jules Levin
ICAAC 2014 Sep 5-9 Wash DC
D Ando1,J Lalezari2, G Blick3, R Hsu4, E DeJesus5, T Hawkins6, J Morales-Ramirez7, D Parks8, W Tang1, S Wang1, G Lee1, G Nichol1, J Zeidan9, RP Sekaly9, R Mitsuyasu10
1Sangamo BioSciences, Richmond, CA;2Quest ClinRes, San Francisco, CA; 3Circle Care, Norwalk, CT; 4 NY, NY; 5OIC, Orlando FL; 6SW Care, Santa Fe, NM; 7Clin Res Ctr, San Juan, PR; 8Central West ClinRes, St Louis, MO; 9VGTI Florida, Port Saint Lucie, FL;10UCLA, Los Angeles, CA
Program Abstract:
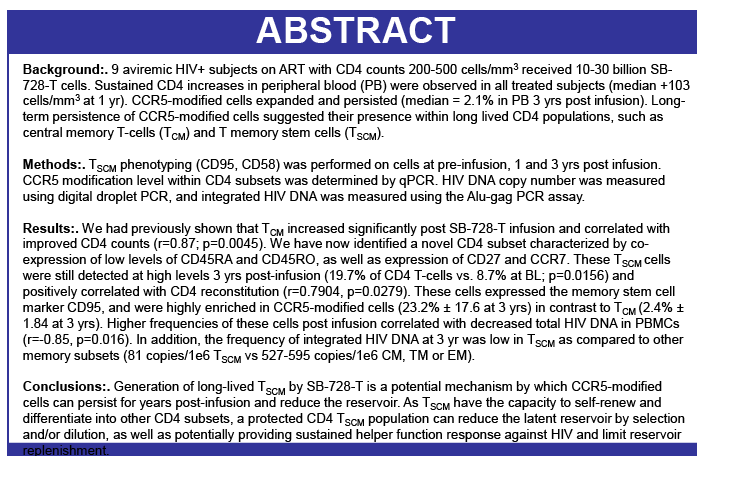
Background: SB-728-T treatment is associated with CD4 reconstitution and reductions in proviral DNA. SB-728-T administered to a single CCR5 ∼32 heterozygote resulted in short-term control of HIV, possibly due to increased frequency of CCR5 bi-allelic modified cells. We performed a prospective study in HIV infected CCR5 ∼32 heterozygotes to confirm this finding. A second study was performed in adults pre-treated with i.v. Cytoxan in order to enhance engraftment. Methods: Two prospective studies were performed on HIV-infected adults: (1) CCR5 ∼32 heterozygotes (n=10), and (2) CCR5 wildtype (n=12) conditioned with Cytoxan (0.1-1 g/m2). Standard clinical and laboratory safety was monitored; HIV VL, HIV-DNA in PBMC (by digital droplet PCR), CD8 anti-GAG intracellular cytokine staining and SB-728-T modified cell number (by qPCR) were measured. Results: VL decreased >1 log from peak in 3 of 7 ∼32 HIV subjects during TI. Two subjects achieved VL below the limit of quantification, continuing in one subject for 21 weeks with ongoing control thereafter (between 64-260 copies/mL for 24 weeks and ongoing). Reduction from peak VL correlated with the level of circulating CCR5 bi-allelic modified cells during TI for the 5 subjects who completed TI (R=-0.90, p=0.037). Subjects with reduced VL, during TI, had both a low proviral DNA level at baseline (mean=63±51 copies/ 106 PBMC) and enhanced CD8 anti-GAG responses after SB-728-T infusion. Cytoxan conditioning was well tolerated from 0.1-1 g/m2 with dose related increases in total and CCR5-modified CD4 T-cells. Three of nine subjects had an ~1 to >2 log decrease in VL during TI. Conclusions: High levels of CCR5 modification, CD8 anti-GAG responses, and low HIV proviral DNA in PBMCs appear to play an important role in functional control of HIV with SB-728-T treatment. Cytoxan conditioning appears generally safe and well tolerated up to a 1 g/m2 dose, and provides enhanced SB-728-T engraftment as well as improved total CD4 T cells. These results support further studies of immune effects of SB-728-T on acute VL and HIV reservoir.

-----------------------------------------
Adoptive Transfer of ZFN CCR5 Modified CD4 T-cells (SB-728-T) in HIV Subjects Leads to Generation of T Memory Stem Cells With a Small Reservoir Size
Reported by Jules Levin
ICAAC 2014 Wash DC Sept 5-9
Joumana Zeidan1, Gary Lee4, RemiFromentin1, Jay Lalezari2, Ronald Mitsuyasu3, Shelley Wang4, Marty Giedlin4, Geoff Nichol4, WinsonTang4, Nicolas Chomont1, Dale Ando4, Rafick-Pierre Sekaly1
1VGTI Florida, Port St Lucie, FL; 2Quest Clinical Research., San Francisco, CA; 3UCLA, Los Angeles, CA; and 4Sangamo BioSciences, Richmond, CA

|
| |
|
 |
 |
|
|