 |
 |
 |
| |
TDF/FTC Vaginal Ring Protects 6 of 6 Monkeys From Weekly SHIV Exposure
|
| |
| |
ICAAC 2014. September 5-9, 2014. Washington, DC
Mark Mascolini
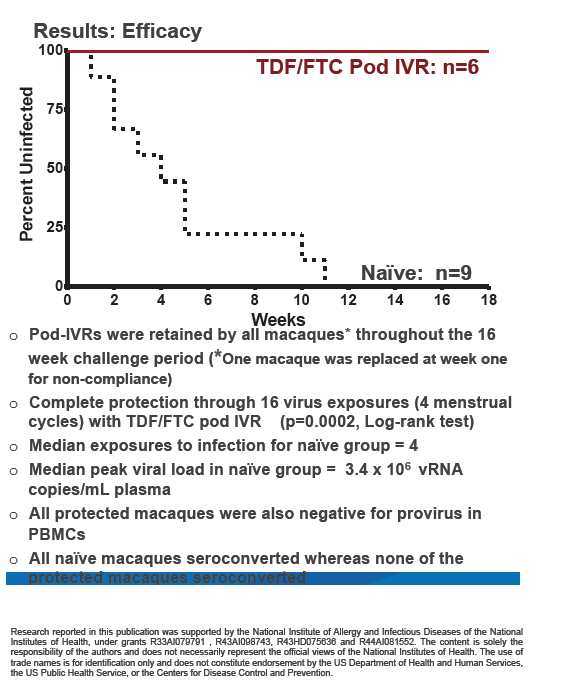
Intravaginal rings bearing tenofovir and emtricitabine (TDF/FTC) in six pods protected 6 of 6 macaques from weekly exposure to simian HIV (SHIV), while all animals not wearing a ring became infected [1]. This study by CDC researchers and collaborators suggests a preexposure prophylaxis (PrEP) approach with minimal adherence requirements.
Four placebo-controlled trials show that oral TDF and TDF/FTC protect people from HIV infection, as long as they take the pills regularly. Poor adherence in two other trials largely explained failure of oral TDF/FTC to protect high-risk women from HIV. An intravaginal ring that releases antiretrovirals for weeks requires only the adherence needed to insert the ring at regular intervals.
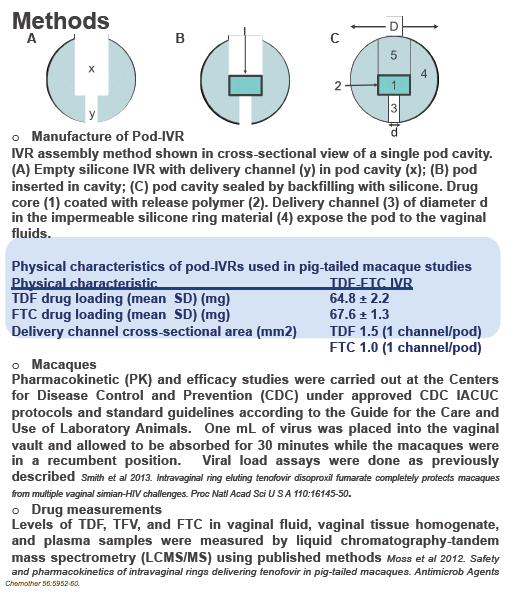
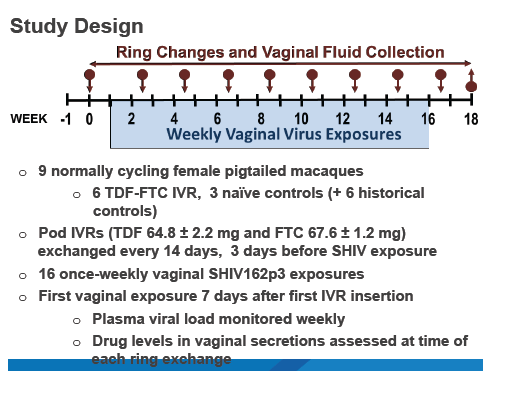
The CDC study involved 9 normally cycling female macaques, 6 of them fitted with pod intravaginal rings loaded with 66 mg of TDF and 68 mg of FTC. Each ring contains six pods, three filled with TDF and three with FTC. Three macaques did not get rings.
The researchers placed rings 1 week before vaginal exposure to 50 TCID50 of SHIV162pd. Exposures continued weekly for 16 weeks or until a macaque became infected. Researchers replaced the TDF/FTC ring every 2 weeks, 3 days before SHIV exposure. They measured plasma viral load weekly and compared SHIV infection rates in macaques wearing rings, the 3 real-time controls not wearing rings, and 6 historical controls not wearing rings. The investigators measured drug levels in vaginal secretions at each ring exchange.
The CDC team replaced 1 macaque 1 week into the study because of "noncompliance." After that change, all 6 macaques retained their rings through the 16-week challenge period. All control animals without intravaginal rings became infected after a median of 4 SHIV exposures. In infected animals, viral load peaked at a median of 3.4 million copies/mL of plasma.
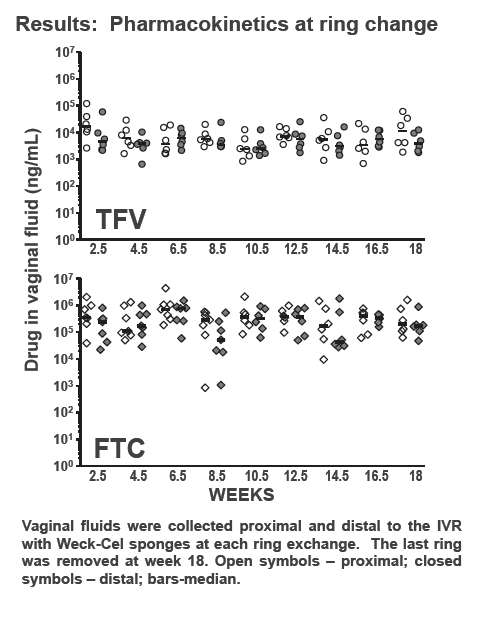
Viral RNA testing showed that none of the 6 macaques wearing rings became infected through 16 weekly SHIV exposures over four menstrual cycles (P = 0.0002 versus controls). No ring-wearing animals had detectable provirus in peripheral blood mononuclear cells. In 28-day pharmacokinetic analyses, median tenofovir and FTC exposures were 0.68 +/- 0.26 mg/day and 2.50 +/- 0.58 mg/day.
The researchers concluded that pod intravaginal rings efficiently release TDF and FTC to maintain protective levels through four menstrual cycles. A 10-pod human-size ring could hold a total of 2 g of drug, which could allow development of rings that last 28 days or longer. The CDC investigators proposed that this PrEP strategy "provides complete protection from SHIV infection in a rigorous animal model and does not require adherence to daily dosing." They called for clinical evaluation of TDF/FTC pod rings in women.
Reference
1. Smith JM, Srinivasan P, Moss JA, et al. Complete protection in macaques from multiple simian-human immunodeficiency virus exposures with pod-intravaginal rings delivering an antiretroviral combination. ICAAC 2014. September 5-9, 2014. Washington, DC. Abstract H-998.
--------------------

|
| |
|
 |
 |
|
|