 |
 |
 |
| |
A 28-Day High-Dose Safety and Pharmacokinetics
Study of Raltegravir in Healthy Subjects
|
| |
| |
Reported by Jules Levin
IDSA 2014, October 8-12, 2014, Philadelphia
Krishna R1, Rizk ML1, Schulz V1, Bruggencate-Broeders ten J1, Liu R1, Larson P1, and Farha KA2
1Merck Research Laboratories, Merck & Co., Inc., Whitehouse Station, NJ, US; 2QPS Netherlands
ONCEMRK - First Patient Enrolled in New Phase 3 Trial Program Investigating a Once-Daily Dosing Regimen of ISENTRESS® (raltegravir).....http://www.natap.org/2014/HIV/060914_11.htm
CROI/2014: A Multiple Dose Study of Raltegravir Formulations....http://www.natap.org/2014/CROI/croi_200.htm
14th European AIDS Conference. October 16-19, 2013. Brussels EACS: A Single Dose Food Effect Study of Raltegravir (RAL) Formulations Once Daily Tablet - (10/17/13)
EACS: Once-Daily Raltegravir for 48 Weeks as Maintenance Therapy in Paris - (10/21/13)
EACS: Merck Presents New Pharmacokinetic Data on ISENTRESS® (raltegravir) at 14th European AIDS Conference - (10/25/13)
15th Pharmacology WK/2014 - Raltegravir Levels With Once-Daily Dosing in French Observational Study in Patients with Suppressed Viremia....http://www.natap.org/2014/Pharm/Pharm_45.htm
Pharmacokinetics and Pharmacogenomics of Once-Daily Raltegravir and Atazanavir in Healthy Volunteers.....http://www.natap.org/2012/HIV/122012_02.htm
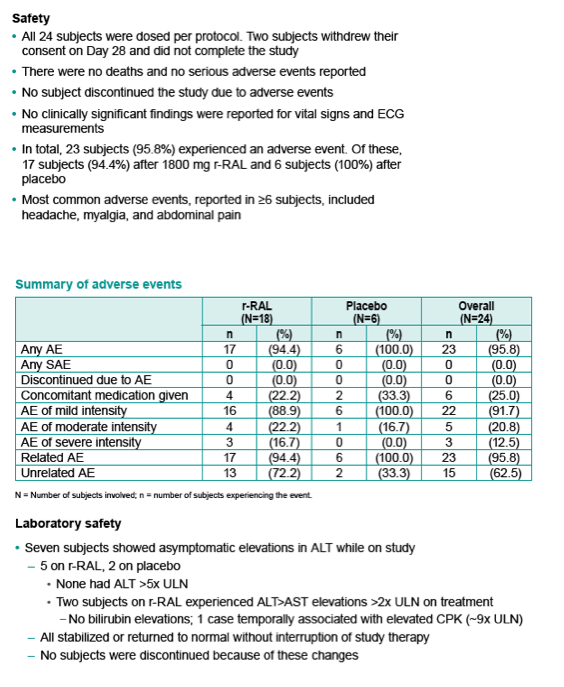
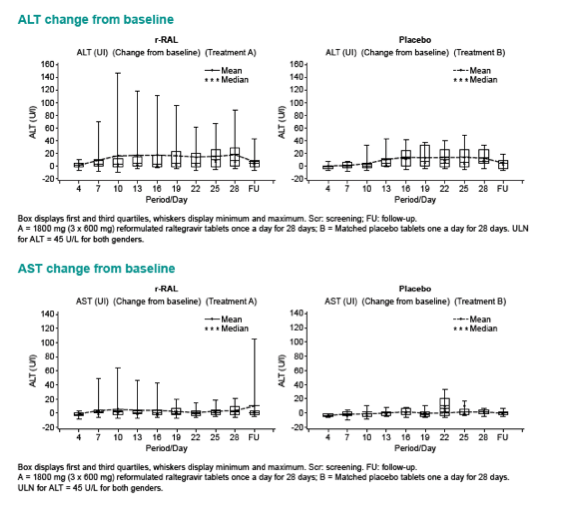
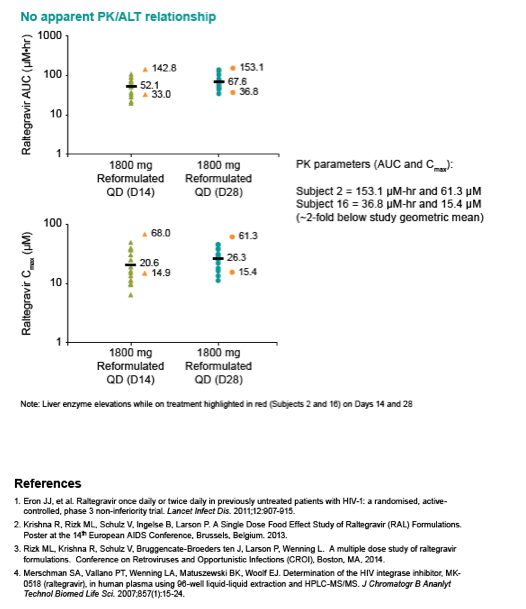
|
| |
|
 |
 |
|
|