 |
 |
 |
| |
Population PK-PD Analysis of 400 mg vs. 600 mg Efavirenz Once Daily in Treatment-Naïve HIV Patients at 48 Weeks: Results of the ENCORE1 Study
|
| |
| |
Reported by Jules Levin
15th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy
Washington DC, USA. 19 May 2014
15th Intl Wrkshp Clinical Pharm HIV Therapy Efavirenz Dose and Genotype Do Not Affect 48-Week Response in Low/Standard-Dose Trial - written by Mark Mascolini - (05/20/14)
Laura Dickinson1, Janaki Amin2, Laura Else1, Deirdre Egan1, Andrew Owen1, Saye Khoo1, David Back1, David A Cooper2, Sean Emery2, Rebekah Puls2, on behalf of the ENCORE1 study group
1 Department of Molecular & Clinical Pharmacology, University of Liverpool, Liverpool, UK; 2 The Kirby Institute, UNSW Australia, Sydney, Australia
Program Abstract:
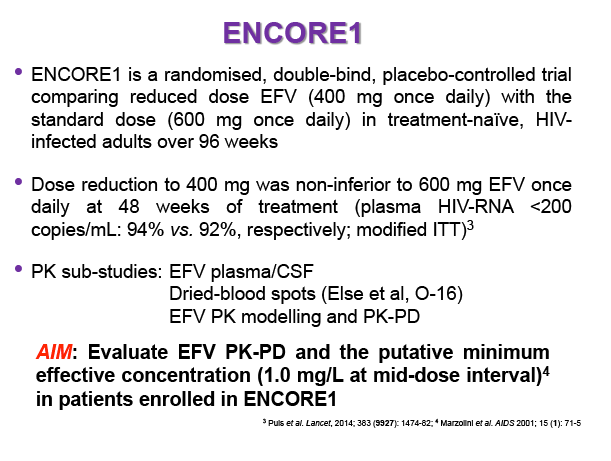
Population PK-PD analysis of 400mg vs. 600mg efavirenz (EFV) once daily in treatment-naïve HIV patients at 48 weeks: results of the ENCORE1 study
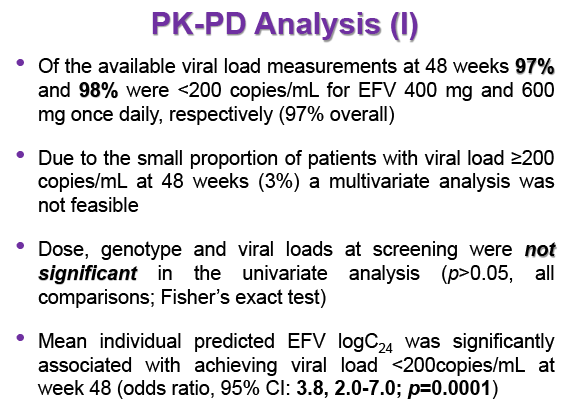
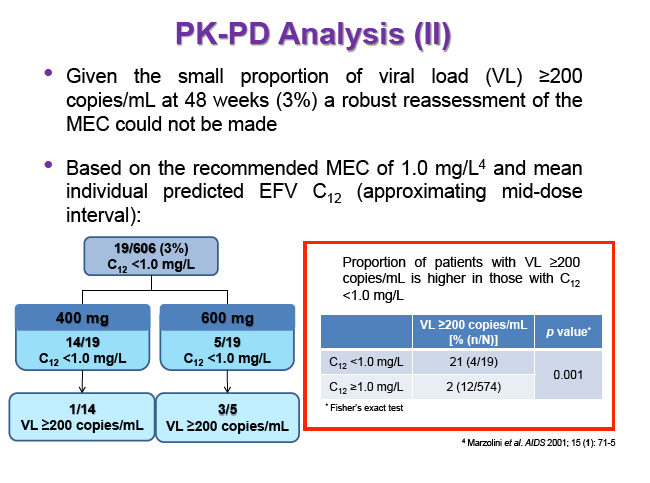
Background: Dose reduction strategies that maintain therapeutic success may decrease costs and reduce drug-related toxicities. Reduction of EFV to 400mg once daily showed non-inferior viral suppression (<200copies/mL) with the standard 600mg dose at 48 weeks in treatment-naïve patients in the ENCORE1 study. The present analysis sought to evaluate EFV PK-PD and the putative minimum effective concentration (MEC ≥1.0mg/L at mid-dose interval) in ENCORE1 patients.
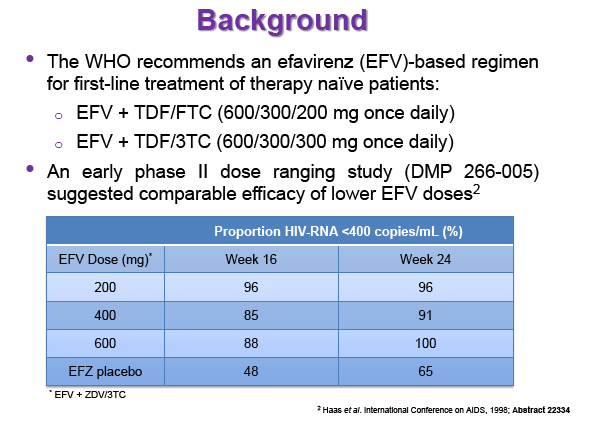
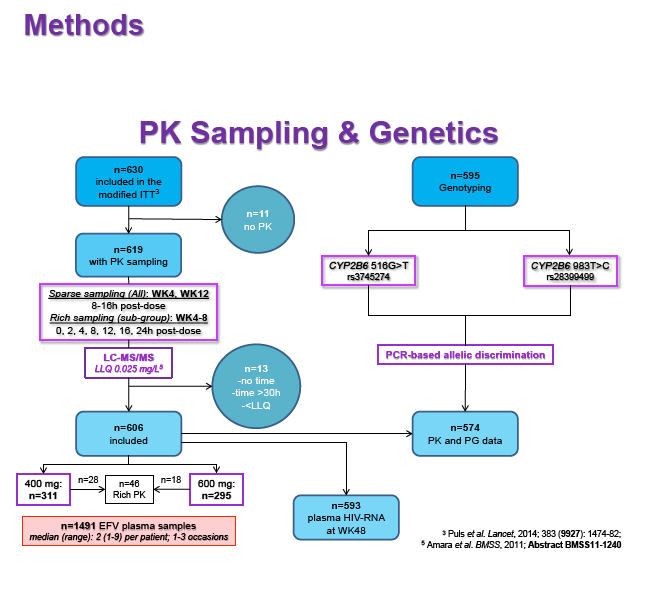
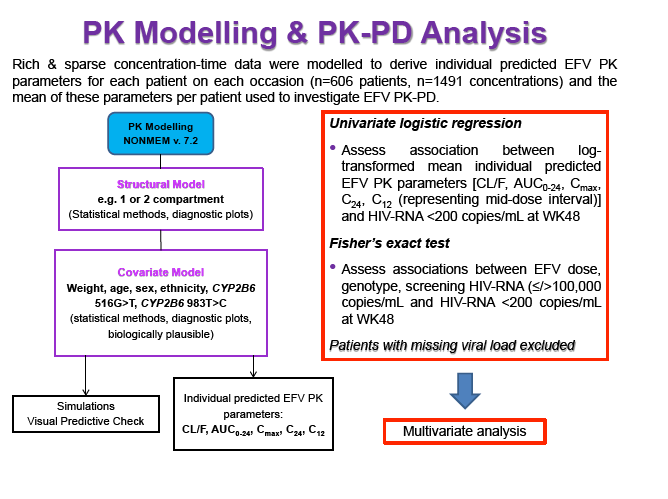
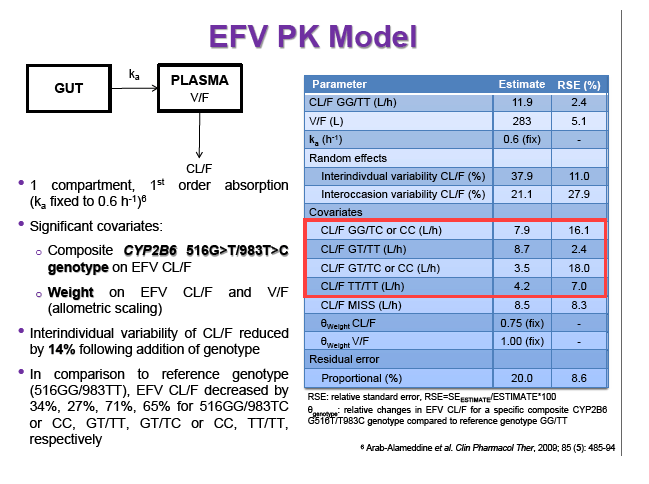
Materials & Methods: Patients were randomised to receive 400mg or 600mg EFV with tenofovir disoproxil fumarate/emtricitabine (300/200mg) once daily. Single blood samples were drawn 8-16h post-dose on weeks 4 and 12 and additional intensive sampling also performed in a sub-group of patients (4-8 weeks). Plasma concentrations were quantified by validated LC-MS/MS and genotyping conducted using TaqMan assays and PCR-based allelic discrimination. Nonlinear mixed effects modelling was applied (NONMEM v. 7.2) to estimate EFV PK parameters and variability. Covariates weight, age, sex, ethnicity and CYP2B6 516G>T and 983T>C genotypes were investigated. Associations between log-transformed mean individual predicted EFV PK parameters [CL/F, AUC0-24, Cmax, trough concentration (C24), concentration 12h post-dose (C12) representing mid-dose interval] and plasma viral load (VL) <200copies/mL at 48 weeks were evaluated by logistic regression. Categorical covariates (EFV dose, genotype and screening VL ≤/>100,000copies/mL) were assessed by exact chi-square. Missing VLs were excluded.
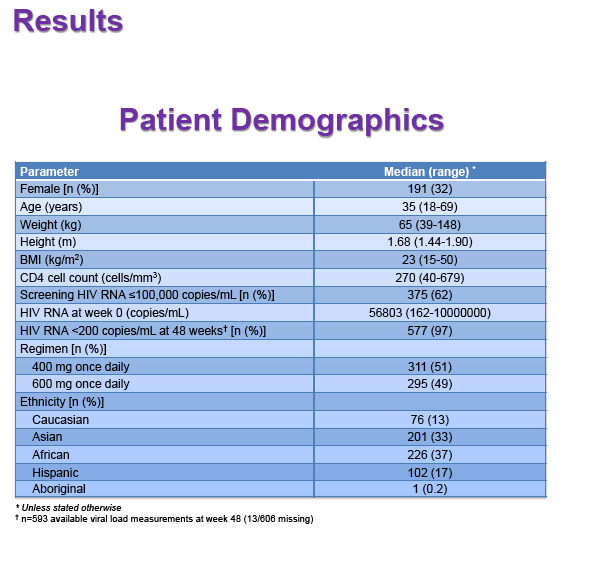
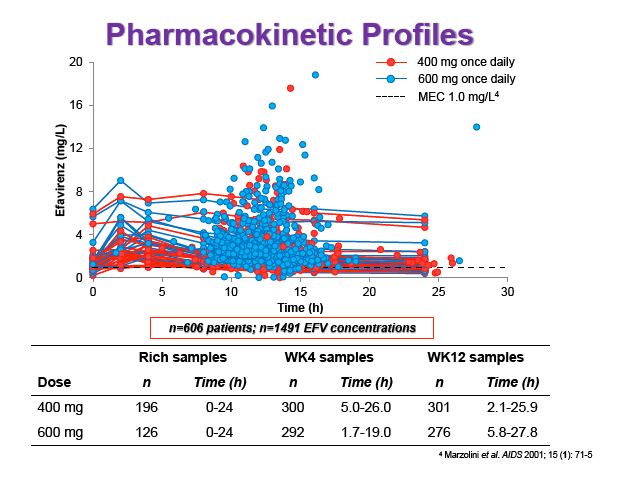
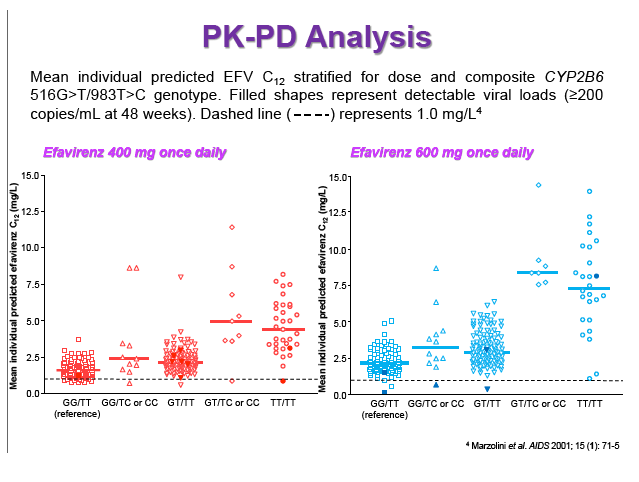
Results: 606 patients (32% female, 30% Caucasian, 33% Asian, 37% African) were included (400mg, n=311; 600mg n=295; 46/606 also had intensive sampling), providing 1491 samples in total (1-9 samples/patient; 1-3 occasions). Median (range) age, weight, baseline CD4 and VL were 35yr (18-69), 65kg (39-148), 270cells/mm3 (40-679) and 56,803copies/mL (162-10,000,000), respectively. Genotypes were available for 574 patients. A one-compartment model best described the data with CYP2B6 G516T/T983C composite genotype (defined below) and weight significantly associated with EFV CL/F (interindividual and interoccasion variability: 38% and 21%, respectively). EFV CL/F decreased by 34, 27, 71 and 65% for 516GG/983TC or CC, GT/TT, GT/TC or CC and TT/TT, respectively compared to wild-type (GG/TT). At 48 weeks 97% of patients with available VL achieved <200copies/mL (97% vs. 98% for 400mg and 600mg EFV, respectively). Of the PK parameters assessed, logC24 was associated with VL <200copies/mL at 48 weeks (odds ratio, 95% CI: 3.1, 1.6-5.9; p=0.001). Due to the low proportion of virological failures, multivariate analysis was not feasible; however dose, genotype and screening VL were not significant in univariate analyses (chi-square; p>0.05 all comparisons). In participants with C12 <1.0mg/L and ≥1.0mg/L, incidence of VL ≥200copies/mL was 21% and 2% at 48 weeks, respectively (chi-square; p=0.001). Of the 4 participants with both VL ≥200 copies/mL and C12 <1.0mg/L, 1 and 3 received 400mg and 600mg EFV, respectively.
Conclusions: Virological efficacy was comparable between doses despite lower concentrations in the 400mg EFV arm. Relationships between CYP2B6 polymorphisms and weight with EFV PK were consistent with previous reports. The number of patients with VL ≥200copies/mL at 48 weeks were very low, although higher in those with EFV C12 < MEC (1.0mg/L). As virological outcomes were not related to dose allocation, other drivers of virological response such as adherence may be involved.


|
| |
|
 |
 |
|
|